Role of probiotics in prevention and treatment of enteric infections: a comprehensive review
- PMID: 33968585
- PMCID: PMC8079594
- DOI: 10.1007/s13205-021-02796-7
Role of probiotics in prevention and treatment of enteric infections: a comprehensive review
Abstract
Microorganisms that inhabits human digestive tract affect global health and enteric disorders. Previous studies have documented the effectiveness and mode of action of probiotics and classified as human-friendly biota and a competitor to enteric pathogens. Statistical studies reported more than 1.5 billion cases of gastrointestinal infections caused by enteric pathogens and their long-term exposure can lead to mental retardation, temporary or permanent physical weakness, and leaving the patient susceptible for opportunistic pathogens, which can cause fatality. We reviewed previous literature providing evidence about therapeutic approaches regarding probiotics to cure enteric infections efficiently by producing inhibitory substances, immune system modulation, improved barrier function. The therapeutic effects of probiotics have shown success against many foodborne pathogens and their therapeutic effectiveness has been exponentially increased using genetically engineered probiotics. The bioengineered probiotic strains are expected to provide a better and alternative approach than traditional antibiotic therapy against enteric pathogens, but the novelty of these strains also raise doubts about the possible untapped side effects, for which there is a need for further studies to eliminate the concerns relating to the use and safety of probiotics. Many such developments and optimization of the classical techniques will revolutionize the treatments for enteric infections.
Keywords: Bacterial strains; Enteric infections; Probiotics; Regulation of gut barrier; Therapeutic approach.
© King Abdulaziz City for Science and Technology 2021.
Figures
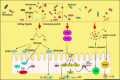
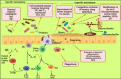




Similar articles
-
Probiotic engineering: towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens.Gut Pathog. 2017 May 8;9:28. doi: 10.1186/s13099-017-0178-9. eCollection 2017. Gut Pathog. 2017. PMID: 28491143 Free PMC article. Review.
-
Bioengineered probiotics, a strategic approach to control enteric infections.Bioengineered. 2013 Nov-Dec;4(6):379-87. doi: 10.4161/bioe.23574. Epub 2013 Jan 17. Bioengineered. 2013. PMID: 23327986 Free PMC article. Review.
-
Antivirulence Properties of Probiotics in Combating Microbial Pathogenesis.Adv Appl Microbiol. 2017;98:1-29. doi: 10.1016/bs.aambs.2016.12.001. Epub 2017 Jan 28. Adv Appl Microbiol. 2017. PMID: 28189153 Review.
-
Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges.Crit Rev Food Sci Nutr. 2019;59(20):3320-3333. doi: 10.1080/10408398.2018.1490885. Epub 2018 Sep 5. Crit Rev Food Sci Nutr. 2019. PMID: 29993263 Review.
-
Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria.Gut Microbes. 2020 Nov 9;12(1):1831339. doi: 10.1080/19490976.2020.1831339. Gut Microbes. 2020. PMID: 33112695 Free PMC article. Review.
Cited by
-
Harnessing the probiotic properties and immunomodulatory effects of fermented food-derived Limosilactobacillus fermentum strains: implications for environmental enteropathy.Front Nutr. 2023 Jun 5;10:1200926. doi: 10.3389/fnut.2023.1200926. eCollection 2023. Front Nutr. 2023. PMID: 37342549 Free PMC article.
-
Beneficial Effects of Probiotics on Benign Gynaecological Disorders: A Review.Nutrients. 2023 Jun 13;15(12):2733. doi: 10.3390/nu15122733. Nutrients. 2023. PMID: 37375637 Free PMC article. Review.
-
The Yin and Yang of pathogens and probiotics: interplay between Salmonella enterica sv. Typhimurium and Bifidobacterium infantis during co-infection.Front Microbiol. 2024 May 15;15:1387498. doi: 10.3389/fmicb.2024.1387498. eCollection 2024. Front Microbiol. 2024. PMID: 38812689 Free PMC article.
-
Interactions between Dietary Antioxidants, Dietary Fiber and the Gut Microbiome: Their Putative Role in Inflammation and Cancer.Int J Mol Sci. 2024 Jul 28;25(15):8250. doi: 10.3390/ijms25158250. Int J Mol Sci. 2024. PMID: 39125822 Free PMC article. Review.
-
A systematic review on selection characterization and implementation of probiotics in human health.Food Sci Biotechnol. 2023 Jan 10;32(4):423-440. doi: 10.1007/s10068-022-01210-z. eCollection 2023 Mar. Food Sci Biotechnol. 2023. PMID: 36911328 Free PMC article. Review.
References
-
- Abou-Kassem DE, Elsadek MF, Abdel-Moneim AE, Mahgoub SA, Elaraby GM, Taha AE, Ashour EA. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum) Poul Sci. 2021;100(1):84–93. - PMC - PubMed
-
- Aguilar C, Vanegas C, Klotz B. Antagonistic effect of Lactobacillus strains against Escherichia coli and Listeria monocytogenes in milk. J Dairy Res. 2011;78(2):136. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

