Narrative review of urinary glycan biomarkers in prostate cancer
- PMID: 33968674
- PMCID: PMC8100853
- DOI: 10.21037/tau-20-964
Narrative review of urinary glycan biomarkers in prostate cancer
Abstract
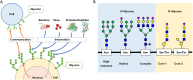
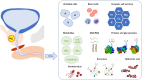
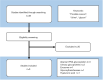
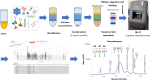
Prostate cancer (PC) is the second most common cancer in men worldwide. The application of the prostate-specific antigen (PSA) test has improved the diagnosis and treatment of PC. However, the PSA test has become associated with overdiagnosis and overtreatment. Therefore, there is an unmet need for novel diagnostic, prognostic, and predictive biomarkers of PC. Urinary glycoproteins and exosomes are a potential source of PC glycan biomarkers. Urinary glycan profiling can provide noninvasive monitoring of tumor heterogeneity and aggressiveness throughout a treatment course. However, urinary glycan profiling is not popular due to technical disadvantages, such as complicated structural analysis that requires specialized expertise. The technological development of glycan analysis is a rapidly advancing field. A lectin-based microarray can detect aberrant glycoproteins in urine, including PSA glycoforms and exosomes. Glycan enrichment beads can enrich the concentration of N-linked glycans specifically. Capillary electrophoresis, liquid chromatography-tandem mass spectrometry, and matrix-assisted laser desorption/ionization-time of flight mass spectrometry can detect glycans directory. Many studies suggest potential of urinary glycoproteins, exosomes, and glycosyltransferases as a biomarker of PC. Although further technological challenges remain, urinary glycan analysis is one of the promising approaches for cancer biomarker discovery.
Keywords: Prostate cancer (PC); biomarker; glycan; glycosylation; urinary.
2021 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-964). The series “Urinary Biomarkers of Urothelial Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Figures

Similar articles
-
Lectin Affinity Chromatography for the Discovery of Novel Cancer Glycobiomarkers: A Case Study with PSA Glycoforms and Prostate Cancer.Methods Mol Biol. 2022;2370:301-313. doi: 10.1007/978-1-0716-1685-7_15. Methods Mol Biol. 2022. PMID: 34611876
-
Profiling the proteoforms of urinary prostate-specific antigen by capillary electrophoresis - mass spectrometry.J Proteomics. 2021 Apr 30;238:104148. doi: 10.1016/j.jprot.2021.104148. Epub 2021 Feb 19. J Proteomics. 2021. PMID: 33618028
-
N-linked (N-) glycoproteomics of urinary exosomes. [Corrected].Mol Cell Proteomics. 2015 Feb;14(2):263-76. doi: 10.1074/mcp.M114.040345. Epub 2014 Dec 1. Mol Cell Proteomics. 2015. PMID: 25452312 Free PMC article.
-
Recent progress and perspectives on prostate cancer biomarkers.Int J Clin Oncol. 2017 Apr;22(2):214-221. doi: 10.1007/s10147-016-1049-y. Epub 2016 Oct 11. Int J Clin Oncol. 2017. PMID: 27730440 Free PMC article. Review.
-
Approaches to the discovery of non-invasive urinary biomarkers of prostate cancer.Oncotarget. 2018 Aug 21;9(65):32534-32550. doi: 10.18632/oncotarget.25946. eCollection 2018 Aug 21. Oncotarget. 2018. PMID: 30197761 Free PMC article. Review.
Cited by
-
Glyco-signatures in patients with advanced lung cancer during anti-PD-1/PD-L1 immunotherapy.Acta Biochim Biophys Sin (Shanghai). 2024 Jun 28;56(8):1099-1107. doi: 10.3724/abbs.2024110. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38952341 Free PMC article.
-
Direct N-Glycosylation Profiling of Urine and Prostatic Fluid Glycoproteins and Extracellular Vesicles.Front Chem. 2021 Sep 27;9:734280. doi: 10.3389/fchem.2021.734280. eCollection 2021. Front Chem. 2021. PMID: 34646811 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2021-2022.Mass Spectrom Rev. 2025 May-Jun;44(3):213-453. doi: 10.1002/mas.21873. Epub 2024 Jun 24. Mass Spectrom Rev. 2025. PMID: 38925550 Free PMC article. Review.
-
The research progress on diagnostic indicators related to prostate-specific antigen gray-zone prostate cancer.BMC Cancer. 2025 Aug 4;25(1):1264. doi: 10.1186/s12885-025-14505-1. BMC Cancer. 2025. PMID: 40760471 Free PMC article. Review.
-
Recent Progress in Biosensors for Detection of Tumor Biomarkers.Molecules. 2022 Oct 28;27(21):7327. doi: 10.3390/molecules27217327. Molecules. 2022. PMID: 36364157 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
