Pre-Immunotherapy Contrast-Enhanced CT Texture-Based Classification: A Useful Approach to Non-Small Cell Lung Cancer Immunotherapy Efficacy Prediction
- PMID: 33968716
- PMCID: PMC8103028
- DOI: 10.3389/fonc.2021.591106
Pre-Immunotherapy Contrast-Enhanced CT Texture-Based Classification: A Useful Approach to Non-Small Cell Lung Cancer Immunotherapy Efficacy Prediction
Abstract
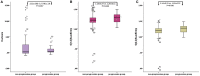
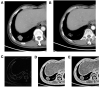
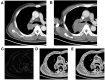
Objective: To investigate the utility of the pre-immunotherapy contrast-enhanced CT-based texture classification in predicting response to non-small cell lung cancer (NSCLC) immunotherapy treatment. Methods: Sixty-three patients with 72 lesions who received immunotherapy were enrolled in this study. We extracted textures including histogram, absolute gradient, run-length matrix, gray-level co-occurrence matrix, autoregressive model, and wavelet transform from pre-immunotherapy contrast-enhanced CT by using Mazda software. Three different methods, namely, Fisher coefficient, mutual information measure (MI), and minimization of classification error probability combined average correlation coefficients (POE + ACC), were performed to select 10 optimal texture feature sets, respectively. The patients were divided into non-progressive disease (non-PD) and progressive disease (PD) groups. t-test or Mann-Whitney U-test was performed to test the differences in each texture feature set between the above two groups. Each texture feature set was analyzed by principal component analysis (PCA), linear discriminant analysis (LDA), and non-linear discriminant analysis (NDA). The area under the curve (AUC) was used to quantify the predictive accuracy of the above three analysis models for each texture feature set, and the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) were also calculated, respectively. Results: Among the three texture feature sets, the texture parameter differences of kurtosis (2.12 ± 3.92 vs. 0.78 ± 1.10, p = 0.047), "S(2,2)SumEntrp" (1.14 ± 0.31 vs. 1.24 ± 0.12, p = 0.036), and "S(1,0)SumEntrp" (1.18 ± 0.27 vs. 1.28 ± 0.11, p = 0.046) between the non-PD and PD group were statistically significant (all p < 0.05). The classification result of texture feature set selected by POE + ACC and analyzed by NDA was identified as the best model (AUC = 0.812, 95% CI: 0.706-0.919) with a sensitivity, specificity, accuracy, PPV, and NPV of 88.2, 76.3, 81.9, 76.9, and 87.9%, respectively. Conclusion: Pre-immunotherapy contrast-enhanced CT-based texture provides a new method for clinical evaluation of the NSCLC immunotherapy efficacy prediction.
Keywords: immunotherapy; non-small cell lung cancer; radiomics; response prediction; texture.
Copyright © 2021 Shen, Fu, Tao, Liu, Yuan and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[Predicting response to non-small cell lung cancer immunotherapy using pre-treatment contrast-enhanced CT texture-based classification].Zhonghua Zhong Liu Za Zhi. 2021 May 23;43(5):541-545. doi: 10.3760/cma.j.cn112152-20190725-00468. Zhonghua Zhong Liu Za Zhi. 2021. PMID: 34034473 Chinese.
-
[Development of a radiomics signature to predict Ki-67 expression level in non-small cell lung cancer].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018 Nov 28;43(11):1216-1222. doi: 10.11817/j.issn.1672-7347.2018.11.008. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 30643066 Chinese.
-
[The value of CT radiomics in the prediction of EGFR mutation in lung cancer].Zhonghua Yi Xue Za Zhi. 2020 Mar 10;100(9):690-695. doi: 10.3760/cma.j.issn.0376-2491.2020.09.009. Zhonghua Yi Xue Za Zhi. 2020. PMID: 32187913 Chinese.
-
Improved window adaptive gray level co-occurrence matrix for extraction and analysis of texture characteristics of pulmonary nodules.Comput Methods Programs Biomed. 2021 Sep;208:106263. doi: 10.1016/j.cmpb.2021.106263. Epub 2021 Jul 3. Comput Methods Programs Biomed. 2021. PMID: 34265545
-
[Texture analysis of diffusion-weighted magnetic resonance imaging to identify atypically enhanced small hepatocellular carcinoma and dysplastic nodules under the background of cirrhosis].Zhonghua Gan Zang Bing Za Zhi. 2020 Jan 20;28(1):37-42. doi: 10.3760/cma.j.issn.1007-3418.2020.01.010. Zhonghua Gan Zang Bing Za Zhi. 2020. PMID: 32023697 Chinese.
Cited by
-
Multiomics-Based Deep Learning Prediction of Prognosis and Therapeutic Response in Patients With Extensive-Stage Small Cell Lung Cancer Receiving Chemoimmunotherapy: A Retrospective Cohort Study.Int J Gen Med. 2025 Feb 24;18:981-996. doi: 10.2147/IJGM.S506485. eCollection 2025. Int J Gen Med. 2025. PMID: 40026810 Free PMC article.
-
Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours.Nat Rev Cancer. 2024 Jul;24(7):498-512. doi: 10.1038/s41568-024-00705-7. Epub 2024 Jun 12. Nat Rev Cancer. 2024. PMID: 38867074 Review.
-
Feature selection methods and predictive models in CT lung cancer radiomics.J Appl Clin Med Phys. 2023 Jan;24(1):e13869. doi: 10.1002/acm2.13869. Epub 2022 Dec 17. J Appl Clin Med Phys. 2023. PMID: 36527376 Free PMC article. Review.
-
Noninvasive assessment of significant liver fibrosis in rabbits by spectral CT parameters and texture analysis.Jpn J Radiol. 2023 Sep;41(9):983-993. doi: 10.1007/s11604-023-01423-0. Epub 2023 Apr 18. Jpn J Radiol. 2023. PMID: 37071251
-
The Role of Radiomics in the Era of Immune Checkpoint Inhibitors: A New Protagonist in the Jungle of Response Criteria.J Clin Med. 2022 Mar 21;11(6):1740. doi: 10.3390/jcm11061740. J Clin Med. 2022. PMID: 35330068 Free PMC article. Review.
References
-
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. 10.1016/S0140-6736(16)32517-X - DOI - PMC - PubMed
-
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. . Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. (2017) 389:67–76. 10.1016/S0140-6736(16)32455-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

