A Novel Role for Brain and Acute Leukemia Cytoplasmic (BAALC) in Human Breast Cancer Metastasis
- PMID: 33968759
- PMCID: PMC8101327
- DOI: 10.3389/fonc.2021.656120
A Novel Role for Brain and Acute Leukemia Cytoplasmic (BAALC) in Human Breast Cancer Metastasis
Abstract
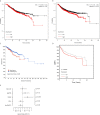
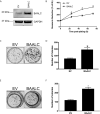
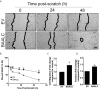
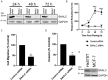
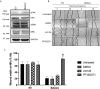
Brain and Acute Leukemia, Cytoplasmic (BAALC) is a protein that controls leukemia cell proliferation, differentiation, and survival and is overexpressed in several cancer types. The gene is located in the chromosomal region 8q22.3, an area commonly amplified in breast cancer and associated with poor prognosis. However, the expression and potential role of BAALC in breast cancer has not widely been examined. This study investigates BAALC expression in human breast cancers with the aim of determining if it plays a role in the pathogenesis of the disease. BAALC protein expression was examined by immunohistochemistry in breast cancer, and matched lymph node and normal breast tissue samples. The effect of gene expression on overall survival (OS), disease-free and distant metastasis free survival (DMFS) was assessed in silico using the Kaplan-Meier Plotter (n=3,935), the TCGA invasive breast carcinoma (n=960) and GOBO (n=821) data sets. Functional effects of BAALC expression on breast cancer proliferation, migration and invasion were determined in vitro. We demonstrate herein that BAALC expression is progressively increased in primary and breast cancer metastases when compared to normal breast tissue. Increased BAALC mRNA is associated with a reduction in DMFS and disease-free survival, but not OS, in breast cancer patients, even when corrected for tumor grade. We show that overexpression of BAALC in MCF-7 breast cancer cells increases the proliferation, anchorage-independent growth, invasion, and migration capacity of these cells. Conversely, siRNA knockdown of BAALC expression in Hs578T breast cancer cells decreases proliferation, invasion and migration. We identify that this BAALC associated migration and invasion is mediated by focal adhesion kinase (FAK)-dependent signaling and is accompanied by an increase in matrix metalloproteinase (MMP)-9 but not MMP-2 activity in vitro. Our data demonstrate a novel function for BAALC in the control of breast cancer metastasis, offering a potential target for the generation of anti-cancer drugs to prevent breast cancer metastasis.
Keywords: BAALC; FAK; breast cancer; invasion; migration.
Copyright © 2021 Birgersson, Chi, Miller, Brzozowski, Brown, Schofield, Taylor, Pearsall, Hewitt, Gedye, Lincz and Skelding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Yucel B, Bahar S, Kacan T, Seker MM, Celasun MG, Bahceci A, et al. . Importance of metastasis site in survival of patients with breast cancer. Austin J Med Oncol (2014) 1:7.
LinkOut - more resources
Full Text Sources
Miscellaneous

