SEMMs: Somatically Engineered Mouse Models. A New Tool for In Vivo Disease Modeling for Basic and Translational Research
- PMID: 33968774
- PMCID: PMC8103029
- DOI: 10.3389/fonc.2021.667189
SEMMs: Somatically Engineered Mouse Models. A New Tool for In Vivo Disease Modeling for Basic and Translational Research
Abstract
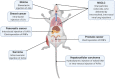
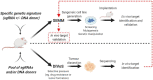
Most experimental oncology therapies fail during clinical development despite years of preclinical testing rationalizing their use. This begs the question of whether the current preclinical models used for evaluating oncology therapies adequately capture patient heterogeneity and response to therapy. Most of the preclinical work is based on xenograft models where tumor mis-location and the lack of the immune system represent a major limitation for the translatability of many observations from preclinical models to patients. Genetically engineered mouse models (GEMMs) hold great potential to recapitulate more accurately disease models but their cost and complexity have stymied their widespread adoption in discovery, early or late drug screening programs. Recent advancements in genome editing technology made possible by the discovery and development of the CRISPR/Cas9 system has opened the opportunity of generating disease-relevant animal models by direct mutation of somatic cell genomes in an organ or tissue compartment of interest. The advent of CRISPR/Cas9 has not only aided in the production of conventional GEMMs but has also enabled the bypassing of the construction of these costly strains. In this review, we describe the Somatically Engineered Mouse Models (SEMMs) as a new category of models where a specific oncogenic signature is introduced in somatic cells of an intended organ in a post-natal animal. In addition, SEMMs represent a novel platform to perform in vivo functional genomics studies, here defined as DIVoS (Direct In Vivo Screening).
Keywords: animal models; clustered regularly interspaced short palindromic repeat/CRISPR associated protein 9-mediated genome editing; genetically engineered mouse models; mouse models; somatically engineered mouse models; translational research.
Copyright © 2021 Lima and Maddalo.
Conflict of interest statement
AL was employed by Genentech, Inc. and DM was employed by Genentech, Inc. and Roche Pharmaceuticals.
Figures
Similar articles
-
Mouse Models for Drug Discovery. Can New Tools and Technology Improve Translational Power?ILAR J. 2016 Dec;57(2):178-185. doi: 10.1093/ilar/ilw021. ILAR J. 2016. PMID: 28053071 Free PMC article.
-
Genetically engineered mouse models for studying radiation biology.Transl Cancer Res. 2017 Jul;6(Suppl 5):S900-S913. doi: 10.21037/tcr.2017.06.19. Transl Cancer Res. 2017. PMID: 30733931 Free PMC article.
-
Drug discovery oncology in a mouse: concepts, models and limitations.Future Sci OA. 2021 Jun 23;7(8):FSO737. doi: 10.2144/fsoa-2021-0019. eCollection 2021 Sep. Future Sci OA. 2021. PMID: 34295539 Free PMC article. Review.
-
Comparative analysis of mouse and human preimplantation development following POU5F1 CRISPR/Cas9 targeting reveals interspecies differences.Hum Reprod. 2021 Apr 20;36(5):1242-1252. doi: 10.1093/humrep/deab027. Hum Reprod. 2021. PMID: 33609360
-
Engineered CRISPR Systems for Next Generation Gene Therapies.ACS Synth Biol. 2017 Sep 15;6(9):1614-1626. doi: 10.1021/acssynbio.7b00011. Epub 2017 Jun 7. ACS Synth Biol. 2017. PMID: 28558198 Review.
Cited by
-
Applications of CRISPR-Cas9 for advancing precision medicine in oncology: from target discovery to disease modeling.Front Genet. 2023 Oct 16;14:1273994. doi: 10.3389/fgene.2023.1273994. eCollection 2023. Front Genet. 2023. PMID: 37908590 Free PMC article. Review.
-
Population-wide gene disruption in the murine lung epithelium via AAV-mediated delivery of CRISPR-Cas9 components.Mol Ther Methods Clin Dev. 2022 Nov 1;27:431-449. doi: 10.1016/j.omtm.2022.10.016. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36419469 Free PMC article.
-
Magnetic resonance imaging and ultrasound elastography in the context of preclinical pharmacological research: significance for the 3R principles.Front Pharmacol. 2023 Jun 28;14:1177421. doi: 10.3389/fphar.2023.1177421. eCollection 2023. Front Pharmacol. 2023. PMID: 37448960 Free PMC article. Review.
-
Transient and DNA-free in vivo CRISPR/Cas9 genome editing for flexible modeling of endometrial carcinogenesis.Cancer Commun (Lond). 2023 May;43(5):620-624. doi: 10.1002/cac2.12409. Epub 2023 Feb 10. Cancer Commun (Lond). 2023. PMID: 36762520 Free PMC article. No abstract available.
-
Mechanisms driving the immunoregulatory function of cancer cells.Nat Rev Cancer. 2023 Apr;23(4):193-215. doi: 10.1038/s41568-022-00544-4. Epub 2023 Jan 30. Nat Rev Cancer. 2023. PMID: 36717668 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

