Reporters of Cancer Stem Cells as a Tool for Drug Discovery
- PMID: 33968778
- PMCID: PMC8100607
- DOI: 10.3389/fonc.2021.669250
Reporters of Cancer Stem Cells as a Tool for Drug Discovery
Abstract
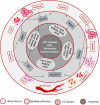
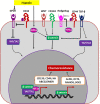
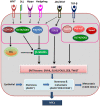
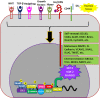
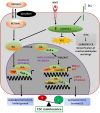
In view of the importance of cancer stem cells (CSCs) in chemoresistance, metastasis and recurrence, the biology of CSCs were explored in detail. Based on that, several modalities were proposed to target them. In spite of the several clinical trials, a successful CSC-targeting drug is yet to be identified. The number of molecules screened and entered for clinical trial for CSC-targeting is comparatively low, compared to other drugs. The bottle neck is the lack of a high-throughput adaptable screening strategy for CSCs. This review is aimed to identify suitable reporters for CSCs that can be used to identify the heterogeneous CSC populations, including quiescent CSCs, proliferative CSCs, drug resistant CSCs and metastatic CSCs. Analysis of the tumor microenvironment regulating CSCs revealed that the factors in CSC-niche activates effector molecules that function as CSC markers, including pluripotency markers, CD133, ABCG2 and ALDH1A1. Among these factors OCT4, SOX2, NANOG, ABCG2 and ALDH1A1 are ideal for making reporters for CSCs. The pluripotency molecules, like OCT4, SOX2 and NANOG, regulate self-renewal, chemoresistance and metastasis. ABCG2 is a known regulator of drug resistance while ALDH1A1 modulates self-renewal, chemoresistance and metastasis. Considering the heterogeneity of CSCs, including a quiescent population and a proliferative population with metastatic ability, we propose the use of a combination of reporters. A dual reporter consisting of a pluripotency marker and a marker like ALDH1A1 will be useful in screening drugs that target CSCs.
Keywords: cancer stem cells; drug resistant CSCs; drug screening and discovery; fluorescent reporters; metastasis initiating cells.
Copyright © 2021 Mohan, Raj R., Mohan, K. P. and Thomas Maliekal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

