The Type II Secretory System Mediates Phage Infection in Vibrio cholerae
- PMID: 33968805
- PMCID: PMC8101328
- DOI: 10.3389/fcimb.2021.662344
The Type II Secretory System Mediates Phage Infection in Vibrio cholerae
Abstract
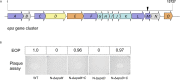
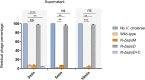
Attachment and specific binding to the receptor on the host cell surface is the first step in the process of bacteriophage infection. The lytic phage VP2 is used in phage subtyping of the Vibrio cholerae biotype El Tor of the O1 serogroup; however, its infection mechanism is poorly understood. In this study, we aimed to identify its receptor on V. cholerae. The outer membrane protein EpsD in the type II secretory system (T2SS) was found to be related to VP2-specific adsorption to V. cholerae, and the T2SS inner membrane protein EpsM had a role in successful VP2 infection, although it was not related to adsorption of VP2. The tail fiber protein gp20 of VP2 directly interacts with EpsD. Therefore, we found that in V. cholerae, in addition to the roles of the T2SS as the transport apparatus of cholera toxin secretion and filamentous phage release, the T2SS is also used as the receptor for phage infection and probably as the channel for phage DNA injection. Our study expands the understanding of the roles of the T2SS in bacteria.
Keywords: EpsD; Vibrio cholerae; bacteriophage; receptor; type II secretory system.
Copyright © 2021 Sun, Liu, Fan, Li, Fan, Zhang, Huang, Li, Li, Xu and Kan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The outer-membrane protein TolC of Vibrio cholerae serves as a second cell-surface receptor for the VP3 phage.J Biol Chem. 2018 Mar 16;293(11):4000-4013. doi: 10.1074/jbc.M117.805689. Epub 2017 Dec 19. J Biol Chem. 2018. PMID: 29259138 Free PMC article.
-
Functional Analysis of Bacteriophage Immunity through a Type I-E CRISPR-Cas System in Vibrio cholerae and Its Application in Bacteriophage Genome Engineering.J Bacteriol. 2015 Nov 23;198(3):578-90. doi: 10.1128/JB.00747-15. Print 2016 Feb 1. J Bacteriol. 2015. PMID: 26598368 Free PMC article.
-
A PolyQ Membrane Protein of Vibrio cholerae Acts as the Receptor for Phage Infection.J Virol. 2021 Feb 24;95(6):e02245-20. doi: 10.1128/JVI.02245-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408174 Free PMC article.
-
CTX prophages in Vibrio cholerae O1 strains.J Microbiol Biotechnol. 2014 Jun 28;24(6):725-31. doi: 10.4014/jmb.1403.03063. J Microbiol Biotechnol. 2014. PMID: 24722374 Review.
-
Cholera toxin phage: structural and functional diversity between Vibrio cholerae biotypes.AIMS Microbiol. 2020 May 28;6(2):144-151. doi: 10.3934/microbiol.2020009. eCollection 2020. AIMS Microbiol. 2020. PMID: 32617446 Free PMC article. Review.
Cited by
-
In through the Out Door: A Functional Virulence Factor Secretion System Is Necessary for Phage Infection in Ralstonia solanacearum.mBio. 2022 Dec 20;13(6):e0147522. doi: 10.1128/mbio.01475-22. Epub 2022 Oct 31. mBio. 2022. PMID: 36314808 Free PMC article.
References
-
- Abendroth J., Rice A. E., McLuskey K., Bagdasarian M., Hol W. G. (2004). The crystal structure of the periplasmic domain of the type II secretion system protein EpsM from Vibrio cholerae: the simplest version of the ferredoxin fold. J. Mol. Biol. 338 (3), 585–596. 10.1016/j.jmb.2004.01.064 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

