Galectin-4 N-Terminal Domain: Binding Preferences Toward A and B Antigens With Different Peripheral Core Presentations
- PMID: 33968903
- PMCID: PMC8097242
- DOI: 10.3389/fchem.2021.664097
Galectin-4 N-Terminal Domain: Binding Preferences Toward A and B Antigens With Different Peripheral Core Presentations
Abstract
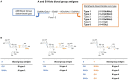
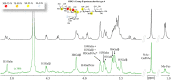
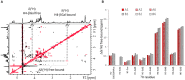
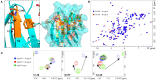
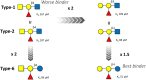
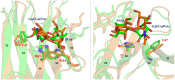
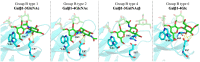
The tandem-repeat Galectin-4 (Gal-4) contains two different domains covalently linked through a short flexible peptide. Both domains have been shown to bind preferentially to A and B histo blood group antigens with different affinities, although the binding details are not yet available. The biological relevance of these associations is unknown, although it could be related to its attributed role in pathogen recognition. The presentation of A and B histo blood group antigens in terms of peripheral core structures differs among tissues and from that of the antigen-mimicking structures produced by pathogens. Herein, the binding of the N-terminal domain of Gal-4 toward a group of differently presented A and B oligosaccharide antigens in solution has been studied through a combination of NMR, isothermal titration calorimetry (ITC), and molecular modeling. The data presented in this paper allow the identification of the specific effects that subtle chemical modifications within this antigenic family have in the binding to the N-terminal domain of Gal-4 in terms of affinity and intermolecular interactions, providing a structural-based rationale for the observed trend in the binding preferences.
Keywords: NMR; blood type antigen; galectin-4; lectin—carbohydrate interaction; molecular recognition.
Copyright © 2021 Quintana, Delgado, Núñez-Franco, Cañada, Jiménez-Osés, Jiménez-Barbero and Ardá.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Different roles of the heterodimer architecture of galectin-4 in selective recognition of oligosaccharides and lipopolysaccharides having ABH antigens.J Biol Chem. 2024 Aug;300(8):107577. doi: 10.1016/j.jbc.2024.107577. Epub 2024 Jul 15. J Biol Chem. 2024. PMID: 39019214 Free PMC article.
-
TF-containing MUC1 glycopeptides fail to entice Galectin-1 recognition of tumor-associated Thomsen-Freidenreich (TF) antigen (CD176) in solution.Glycoconj J. 2020 Dec;37(6):657-666. doi: 10.1007/s10719-020-09951-x. Epub 2020 Oct 1. Glycoconj J. 2020. PMID: 33001366 Free PMC article.
-
Galectin-4 Antimicrobial Activity Primarily Occurs Through its C-Terminal Domain.Mol Cell Proteomics. 2024 May;23(5):100747. doi: 10.1016/j.mcpro.2024.100747. Epub 2024 Mar 13. Mol Cell Proteomics. 2024. PMID: 38490531 Free PMC article.
-
Expression, localization and function of galectin-8, a tandem-repeat lectin, in human tumors.Histol Histopathol. 2014 Sep;29(9):1093-105. doi: 10.14670/HH-29.1093. Epub 2014 Mar 31. Histol Histopathol. 2014. PMID: 24696431 Review.
-
Oligosaccharide specificity of galectins: a search by frontal affinity chromatography.Biochim Biophys Acta. 2002 Sep 19;1572(2-3):232-54. doi: 10.1016/s0304-4165(02)00311-2. Biochim Biophys Acta. 2002. PMID: 12223272 Review.
Cited by
-
Selective 13 C-Labels on Repeating Glycan Oligomers to Reveal Protein Binding Epitopes through NMR: Polylactosamine Binding to Galectins.Angew Chem Int Ed Engl. 2021 Aug 16;60(34):18777-18782. doi: 10.1002/anie.202106056. Epub 2021 Jul 13. Angew Chem Int Ed Engl. 2021. PMID: 34128568 Free PMC article.
-
Galectin-4 levels in hospitalized versus non-hospitalized subjects with obesity: the Malmö Preventive Project.Cardiovasc Diabetol. 2022 Jul 2;21(1):125. doi: 10.1186/s12933-022-01559-9. Cardiovasc Diabetol. 2022. PMID: 35780152 Free PMC article.
-
Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly.Molecules. 2023 May 11;28(10):4039. doi: 10.3390/molecules28104039. Molecules. 2023. PMID: 37241779 Free PMC article.
-
Exploring galectin interactions with human milk oligosaccharides and blood group antigens identifies BGA6 as a functional galectin-4 ligand.J Biol Chem. 2024 Aug;300(8):107573. doi: 10.1016/j.jbc.2024.107573. Epub 2024 Jul 14. J Biol Chem. 2024. PMID: 39009340 Free PMC article.
-
The impact of glycosylation on the structure, function, and interactions of CD14.Glycobiology. 2024 Apr 1;34(3):cwae002. doi: 10.1093/glycob/cwae002. Glycobiology. 2024. PMID: 38227775 Free PMC article.
References
-
- Asensio J. L., Cañada F. J., Jimenez-Barbero J. (1995). Studies of the bound conformations of methyl alpha-lactoside and methyl beta-allolactoside to ricin B chain using transferred NOE experiments in the laboratory and rotating frames, assisted by molecular mechanics and dynamics calculations. Europ. J. Biochem. 233, 618–630. 10.1111/j.1432-1033.1995.618_2.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

