Galectin-4 N-Terminal Domain: Binding Preferences Toward A and B Antigens With Different Peripheral Core Presentations
- PMID: 33968903
- PMCID: PMC8097242
- DOI: 10.3389/fchem.2021.664097
Galectin-4 N-Terminal Domain: Binding Preferences Toward A and B Antigens With Different Peripheral Core Presentations
Abstract
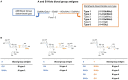
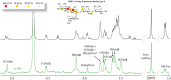
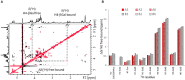
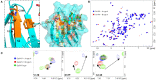
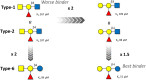
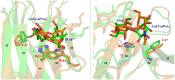
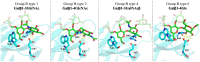
The tandem-repeat Galectin-4 (Gal-4) contains two different domains covalently linked through a short flexible peptide. Both domains have been shown to bind preferentially to A and B histo blood group antigens with different affinities, although the binding details are not yet available. The biological relevance of these associations is unknown, although it could be related to its attributed role in pathogen recognition. The presentation of A and B histo blood group antigens in terms of peripheral core structures differs among tissues and from that of the antigen-mimicking structures produced by pathogens. Herein, the binding of the N-terminal domain of Gal-4 toward a group of differently presented A and B oligosaccharide antigens in solution has been studied through a combination of NMR, isothermal titration calorimetry (ITC), and molecular modeling. The data presented in this paper allow the identification of the specific effects that subtle chemical modifications within this antigenic family have in the binding to the N-terminal domain of Gal-4 in terms of affinity and intermolecular interactions, providing a structural-based rationale for the observed trend in the binding preferences.
Keywords: NMR; blood type antigen; galectin-4; lectin—carbohydrate interaction; molecular recognition.
Copyright © 2021 Quintana, Delgado, Núñez-Franco, Cañada, Jiménez-Osés, Jiménez-Barbero and Ardá.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Asensio J. L., Cañada F. J., Jimenez-Barbero J. (1995). Studies of the bound conformations of methyl alpha-lactoside and methyl beta-allolactoside to ricin B chain using transferred NOE experiments in the laboratory and rotating frames, assisted by molecular mechanics and dynamics calculations. Europ. J. Biochem. 233, 618–630. 10.1111/j.1432-1033.1995.618_2.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

