Recovery of Depleted miR-146a in ALS Cortical Astrocytes Reverts Cell Aberrancies and Prevents Paracrine Pathogenicity on Microglia and Motor Neurons
- PMID: 33968923
- PMCID: PMC8103001
- DOI: 10.3389/fcell.2021.634355
Recovery of Depleted miR-146a in ALS Cortical Astrocytes Reverts Cell Aberrancies and Prevents Paracrine Pathogenicity on Microglia and Motor Neurons
Abstract
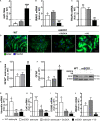
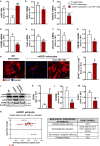
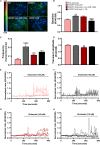
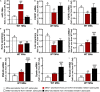
Reactive astrocytes in Amyotrophic Lateral Sclerosis (ALS) change their molecular expression pattern and release toxic factors that contribute to neurodegeneration and microglial activation. We and others identified a dysregulated inflammatory miRNA profile in ALS patients and in mice models suggesting that they represent potential targets for therapeutic intervention. Such cellular miRNAs are known to be released into the secretome and to be carried by small extracellular vesicles (sEVs), which may be harmful to recipient cells. Thus, ALS astrocyte secretome may disrupt cell homeostasis and impact on ALS pathogenesis. Previously, we identified a specific aberrant signature in the cortical brain of symptomatic SOD1-G93A (mSOD1) mice, as well as in astrocytes isolated from the same region of 7-day-old mSOD1 mice, with upregulated S100B/HMGB1/Cx43/vimentin and downregulated GFAP. The presence of downregulated miR-146a on both cases suggests that it can be a promising target for modulation in ALS. Here, we upregulated miR-146a with pre-miR-146a, and tested glycoursodeoxycholic acid (GUDCA) and dipeptidyl vinyl sulfone (VS) for their immunoregulatory properties. VS was more effective in restoring astrocytic miR-146a, GFAP, S100B, HMGB1, Cx43, and vimentin levels than GUDCA, which only recovered Cx43 and vimentin mRNA. The miR-146a inhibitor generated typical ALS aberrancies in wild type astrocytes that were abolished by VS. Similarly, pre-miR-146a transfection into the mSOD1 astrocytes abrogated aberrant markers and intracellular Ca2+ overload. Such treatment counteracted miR-146a depletion in sEVs and led to secretome-mediated miR-146a enhancement in NSC-34-motor neurons (MNs) and N9-microglia. Secretome from mSOD1 astrocytes increased early/late apoptosis and FGFR3 mRNA in MNs and microglia, but not when derived from pre-miR-146a or VS-treated cells. These last strategies prevented the impairment of axonal transport and synaptic dynamics by the pathological secretome, while also averted microglia activation through either secretome, or their isolated sEVs. Proteomic analysis of the target cells indicated that pre-miR-146a regulates mitochondria and inflammation via paracrine signaling. We demonstrate that replenishment of miR-146a in mSOD1 cortical astrocytes with pre-miR-146a or by VS abrogates their phenotypic aberrancies and paracrine deleterious consequences to MNs and microglia. These results propose miR-146a as a new causal and emerging therapeutic target for astrocyte pathogenic processes in ALS.
Keywords: amyotrophic lateral sclerosis; astrocyte-microglia communication; astrocyte-motor neuron crosstalk; calcium signaling aberrancies; glycoursodeoxycholic acid; reactive astrocytes; small extracellular vesicles; vinyl sulfone.
Copyright © 2021 Barbosa, Gomes, Sequeira, Gonçalves-Ribeiro, Pina, Carvalho, Moreira, Vaz, Vaz and Brites.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Neurotoxic Astrocytes Directly Converted from Sporadic and Familial ALS Patient Fibroblasts Reveal Signature Diversities and miR-146a Theragnostic Potential in Specific Subtypes.Cells. 2022 Apr 1;11(7):1186. doi: 10.3390/cells11071186. Cells. 2022. PMID: 35406750 Free PMC article.
-
Astrocyte regional diversity in ALS includes distinct aberrant phenotypes with common and causal pathological processes.Exp Cell Res. 2020 Oct 15;395(2):112209. doi: 10.1016/j.yexcr.2020.112209. Epub 2020 Jul 30. Exp Cell Res. 2020. PMID: 32739211
-
Overexpression of miR-124 in Motor Neurons Plays a Key Role in ALS Pathological Processes.Int J Mol Sci. 2021 Jun 7;22(11):6128. doi: 10.3390/ijms22116128. Int J Mol Sci. 2021. PMID: 34200161 Free PMC article.
-
Significance of aberrant glial cell phenotypes in pathophysiology of amyotrophic lateral sclerosis.Neurosci Lett. 2017 Jan 1;636:27-31. doi: 10.1016/j.neulet.2016.07.052. Epub 2016 Jul 26. Neurosci Lett. 2017. PMID: 27473942 Review.
-
Remodeling of astrocyte secretome in amyotrophic lateral sclerosis: uncovering novel targets to combat astrocyte-mediated toxicity.Transl Neurodegener. 2022 Dec 26;11(1):54. doi: 10.1186/s40035-022-00332-y. Transl Neurodegener. 2022. PMID: 36567359 Free PMC article. Review.
Cited by
-
A microRNA diagnostic biomarker for amyotrophic lateral sclerosis.Brain Commun. 2024 Sep 13;6(5):fcae268. doi: 10.1093/braincomms/fcae268. eCollection 2024. Brain Commun. 2024. PMID: 39280119 Free PMC article.
-
Emerging role of non-coding RNAs in neuroinflammation mediated by microglia and astrocytes.J Neuroinflammation. 2023 Jul 23;20(1):173. doi: 10.1186/s12974-023-02856-0. J Neuroinflammation. 2023. PMID: 37481642 Free PMC article. Review.
-
Neuronal Dynamics and miRNA Signaling Differ between SH-SY5Y APPSwe and PSEN1 Mutant iPSC-Derived AD Models upon Modulation with miR-124 Mimic and Inhibitor.Cells. 2021 Sep 14;10(9):2424. doi: 10.3390/cells10092424. Cells. 2021. PMID: 34572073 Free PMC article.
-
The Double-Edged Effect of Connexins and Pannexins of Glial Cells in Central and Peripheral Nervous System After Nerve Injury.Mol Neurobiol. 2025 May 1. doi: 10.1007/s12035-025-04991-6. Online ahead of print. Mol Neurobiol. 2025. PMID: 40310549 Review.
-
Mesenchymal stem cell-derived extracellular vesicles protect retina in a mouse model of retinitis pigmentosa by anti-inflammation through miR-146a-Nr4a3 axis.Stem Cell Res Ther. 2022 Aug 3;13(1):394. doi: 10.1186/s13287-022-03100-x. Stem Cell Res Ther. 2022. PMID: 35922863 Free PMC article.
References
-
- Almad A. A., Taga A., Joseph J., Welsh C., Patankar A., Gross S. K., et al. (2020). Connexin 43 hemichannels mediate spatial and temporal disease spread in ALS. BioRxiv [Preprint]. 10.1101/2020.03.14.990747 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

