Real-World Methotrexate Dose on Clinical Effectiveness and Structural Damage of Certolizumab Pegol With Rheumatoid Arthritis
- PMID: 33968956
- PMCID: PMC8096982
- DOI: 10.3389/fmed.2021.643459
Real-World Methotrexate Dose on Clinical Effectiveness and Structural Damage of Certolizumab Pegol With Rheumatoid Arthritis
Abstract
Objective: Rheumatoid arthritis (RA) treatments have markedly advanced with the introduction of biological agents, e. g., tumor necrosis factor (TNF) inhibitors. TNF inhibitors are demonstrated to be quite effective in combination with methotrexate (MTX), and sufficient doses of both agents are important to control RA's disease activity. However, not all RA patients can be treated with high-dose MTX due to contraindications related to the antimetabolite action of MTX or to tolerability concerns. In daily practice, this has resulted in reduced effectiveness of TNF inhibitors. We sought to determine whether the concomitant use of dose of MTX affected the clinical effectiveness, retention rate, and side effects of certolizumab pegol (CZP) for treating RA in a real-world setting. CZP is a pegylated-conjugated Fab' fragment of a humanized anti-TNF antibody that has high affinity to TNF. Patients and Methods: We divided Japanese RA patients treated with CZP (n = 95, 25-83 years old) into groups based on those with (n = 65) and without (n = 30) concomitant MTX and those treated with a high dose (≥8 mg, n = 41) or low dose (1- <8 mg, n = 24) of MTX. We retrospectively analyzed the concomitant MTX doses' effects and side effects and the patient retention rate. Results: There were no significant differences among the CZP groups with and without MTX or the groups receiving the high vs. low MTX doses in the retention rate, the low disease activity rate, or the inhibitory effect in radiographic joint damage. Conclusion: CZP has the potential to be a useful biological agent to control RA's disease activity and the bone destruction in patients who cannot tolerate a sufficient MTX dose.
Keywords: DAS28-ESR; X ray; biological; certolizumab pegol; cytokines; rheumatoid arthritis.
Copyright © 2021 Nozaki, Hidaka, Ri, Itami, Tomita, Okada, Ashida, Ikeda, Yamamoto, Funahashi, Kinoshita, Matsubara, Funauchi and Matsumura.
Conflict of interest statement
YN has received honoraria or research grant from AbbVie GK, Astellas Pharma, Asahi Kasei, AYUMI Pharmaceutical, Chugai Pharmaceutical Co., Eisai Co., Daiichi-Sankyo, MSD, Mitsubishi Tanabe Pharma Corp., Takeda, Ono, Otsuka Co., Pfizer, Janssen, and UCB Japan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



 moderate, 3.2 ≤ DAS28 ≤ 5.1;
moderate, 3.2 ≤ DAS28 ≤ 5.1;  low, 2.6 ≤ DAS28 <3.2; □ remission: DAS28 < 2.6. ACR/EULAR Boolean-based remission: TJC, SJC, PtVAS, and CRP all ≤1. CRP, C-reactive protein; EULAR, European League Against Rheumatism; PtVAS, patient's visual analog scale; RA, rheumatoid arthritis; SJC, swelling joint count; TJC, tender joint count.
low, 2.6 ≤ DAS28 <3.2; □ remission: DAS28 < 2.6. ACR/EULAR Boolean-based remission: TJC, SJC, PtVAS, and CRP all ≤1. CRP, C-reactive protein; EULAR, European League Against Rheumatism; PtVAS, patient's visual analog scale; RA, rheumatoid arthritis; SJC, swelling joint count; TJC, tender joint count.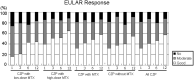
 Moderate response: DAS28 improvement >0.6 and ≤1.2 in present DAS28 ≤3.2, and >3.2 and ≤5.1; DAS28 improvement >1.2 in present DAS28 >5.1, and >3.2 and ≤5.1. □ Good response: DAS28 improvement >1.2 in present DAS28 ≤3.2.
Moderate response: DAS28 improvement >0.6 and ≤1.2 in present DAS28 ≤3.2, and >3.2 and ≤5.1; DAS28 improvement >1.2 in present DAS28 >5.1, and >3.2 and ≤5.1. □ Good response: DAS28 improvement >1.2 in present DAS28 ≤3.2.
References
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. . Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. (2003) 48:35–45. 10.1002/art.10697 - DOI - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. . The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. (2006) 54:26–37. 10.1002/art.21519 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

