Anti-cancer Therapy Leads to Increased Cardiovascular Susceptibility to COVID-19
- PMID: 33969006
- PMCID: PMC8102732
- DOI: 10.3389/fcvm.2021.634291
Anti-cancer Therapy Leads to Increased Cardiovascular Susceptibility to COVID-19
Abstract
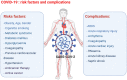
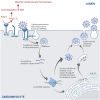
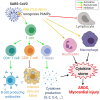
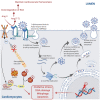
Anti-cancer treatment regimens can lead to both acute- and long-term myocardial injury due to off-target effects. Besides, cancer patients and survivors are severely immunocompromised due to the harsh effect of anti-cancer therapy targeting the bone marrow cells. Cancer patients and survivors can therefore be potentially extremely clinically vulnerable and at risk from infectious diseases. The recent global outbreak of the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its infection called coronavirus disease 2019 (COVID-19) has rapidly become a worldwide health emergency, and on March 11, 2020, COVID-19 was declared a global pandemic by the World Health Organization (WHO). A high fatality rate has been reported in COVID-19 patients suffering from underlying cardiovascular diseases. This highlights the critical and crucial aspect of monitoring cancer patients and survivors for potential cardiovascular complications during this unprecedented health crisis involving the progressive worldwide spread of COVID-19. COVID-19 is primarily a respiratory disease; however, COVID-19 has shown cardiac injury symptoms similar to the cardiotoxicity associated with anti-cancer therapy, including arrhythmia, myocardial injury and infarction, and heart failure. Due to the significant prevalence of micro- and macro-emboli and damaged vessels, clinicians worldwide have begun to consider whether COVID-19 may in fact be as much a vascular disease as a respiratory disease. However, the underlying mechanisms and pathways facilitating the COVID-19-induced cardiac injury in cancer and non-cancer patients remain unclear. Investigations into whether COVID-19 cardiac injury and anti-cancer drug-induced cardiac injury in cancer patients and survivors might synergistically increase the cardiovascular complications and comorbidity risk through a "two-hit" model are needed. Identification of cardiac injury mechanisms and pathways associated with COVID-19 development overlapping with anti-cancer therapy could help clinicians to allow a more optimized prognosis and treatment of cancer survivors suffering from COVID-19. The following review will focus on summarizing the harmful cardiovascular risk of COVID-19 in cancer patients and survivors treated with an anti-cancer drug. This review will improve the knowledge of COVID-19 impact in the field of cardio-oncology and potentially improve the outcome of patients.
Keywords: ACE2; COVID-19; SARS-CoV-2; anti-cancer drug-induced cardiac injury; cytokine storm.
Copyright © 2021 Lozahic, Maddock and Sandhu.
Conflict of interest statement
The handling editor is currently organising a Research Topic with one of the authors HM and confirms the absence of any other collaboration. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

