Paradoxical relationship between proton pump inhibitors and COVID-19: A systematic review and meta-analysis
- PMID: 33969059
- PMCID: PMC8058681
- DOI: 10.12998/wjcc.v9.i12.2763
Paradoxical relationship between proton pump inhibitors and COVID-19: A systematic review and meta-analysis
Abstract
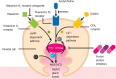
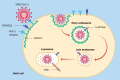
Background: The proton pump inhibitors (PPIs), used to reduce gastric acid secretion, represent one of the most widely used pharmaceutical classes in the world. Their consumption as a risk factor for the evolution of severe forms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been investigated as well as the mortality of these patients. These risks also appear to be linked to the duration and the dosage. On the other hand, several studies have emerged with regard to the protective or therapeutic effects of these drugs. More and more evidence underlines the immunomodulatory and anti-fibrotic role of PPIs. In addition, their ability to alkalize the contents of endosomes and lysosomes serves as an obstacle to the entry of the virus into the host cells.
Aim: To identify studies on the relationship between the intake of PPIs and coronavirus disease 2019 (COVID-19) in patients affected by SARS-CoV-2 infection, with the main objective of evaluating the outcomes related to severity and mortality.
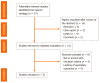
Methods: A literature review was performed in November 2020. The MEDLINE/PubMed, Cochrane Library, EMBASE and Google Scholar databases were searched for all relevant articles published in English on this topic. The search terms were identified by means of controlled vocabularies, such as the National Library of Medicine's MESH (Medical Subject Headings) and keywords. The MESH terms and keywords used were as follows: "COVID-19", "proton pump inhibitors", "PPIs", "SARS-CoV-2", "outcomes", "severity" and "mortality". The inclusion criteria regarding the studies considered in our analysis were: meta-analysis, case-control, hospital-based case-control, population-based case-control, retrospective studies, online survey, as well as cohort-studies, while articles not published as full reports, such as conference abstracts, case reports and editorials were excluded. We tried to summarize and pool all the data if available.
Results: A total of 9 studies were found that described the use of PPIs, of which only 5 clearly reported the severity and mortality data in SARS-CoV-2 patients. Our pooled incidence analysis of severe events did not differ between patients with and without PPIs (odds ratio 1.65, 95% confidence interval: 0.62-4.35) (P = 0.314), or for mortality (odds ratio 1.77, 95% confidence interval: 0.62-5.03) (P = 0.286).
Conclusion: Detailed and larger case studies are needed to accurately understand the role of PPIs in this viral infection.
Keywords: COVID-19; Mortality; Proton pump inhibitors; SARS-CoV-2, Severity.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

