Chemical targets to deactivate biological and chemical toxins using surfaces and fabrics
- PMID: 33969223
- PMCID: PMC8097677
- DOI: 10.1038/s41570-021-00275-4
Chemical targets to deactivate biological and chemical toxins using surfaces and fabrics
Abstract
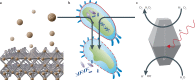
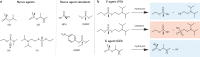
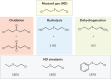
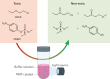
The most recent global health and economic crisis caused by the SARS-CoV-2 outbreak has shown us that it is vital to be prepared for the next global threat, be it caused by pollutants, chemical toxins or biohazards. Therefore, we need to develop environments in which infectious diseases and dangerous chemicals cannot be spread or misused so easily. Especially, those who put themselves in situations of most exposure - doctors, nurses and those protecting and caring for the safety of others - should be adequately protected. In this Review, we explore how the development of coatings for surfaces and functionalized fabrics can help to accelerate the inactivation of biological and chemical toxins. We start by looking at recent advancements in the use of metal and metal-oxide-based catalysts for the inactivation of pathogenic threats, with a focus on identifying specific chemical bonds that can be targeted. We then discuss the use of metal-organic frameworks on textiles for the capture and degradation of various chemical warfare agents and their simulants, their long-term efficacy and the challenges they face.
Keywords: Heterogeneous catalysis; Pollution remediation.
© Springer Nature Limited 2021, corrected publication 2021.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




References
-
- Stevens, P. Diseases of Poverty and the 10/90 Gap (National Prevention Information NetworkBETA, 2004).
-
- Human Wrongs Watch. How air pollution is destroying our health. Human Wrongs Watchhttps://human-wrongs-watch.net/2019/06/04/how-air-pollution-is-destroyin... (2019).
-
- Organisation for the Prohibition of Chemical Weapons. Convention on the Prohibition of the Development, Production and Stockpiling of Chemical Weapons and on their Destruction (2005).
-
- Perper, R. Over 10,000 tear gas canisters have been fired in Hong Kong’s vicious protests, and it could have long-term consequences for the city’s health. Business Insider. https://www.businessinsider.com/experts-long-term-effects-tear-gas-expos... (2020).
-
- Dodd, V., Harding, L. & MacAskill, E. Sergei Skripal: former Russian spy poisoned with nerve agent, say police. The Guardian. https://www.theguardian.com/uk-news/2018/mar/07/russian-spy-police-appea... (2018).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

