Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface
- PMID: 33969332
- PMCID: PMC8084634
- DOI: 10.1016/j.medj.2021.04.016
Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface
Abstract
Background: Pregnant women are at increased risk for severe outcomes from coronavirus disease 2019 (COVID-19), but the pathophysiology underlying this increased morbidity and its potential effect on the developing fetus is not well understood.
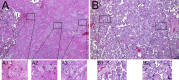
Methods: We assessed placental histology, ACE2 expression, and viral and immune dynamics at the term placenta in pregnant women with and without respiratory severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
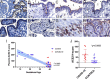
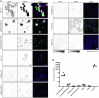
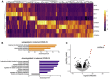
Findings: The majority (13 of 15) of placentas analyzed had no detectable viral RNA. ACE2 was detected by immunohistochemistry in syncytiotrophoblast cells of the normal placenta during early pregnancy but was rarely seen in healthy placentas at full term, suggesting that low ACE2 expression may protect the term placenta from viral infection. Using immortalized cell lines and primary isolated placental cells, we found that cytotrophoblasts, the trophoblast stem cells and precursors to syncytiotrophoblasts, rather than syncytiotrophoblasts or Hofbauer cells, are most vulnerable to SARS-CoV-2 infection in vitro. To better understand potential immune mechanisms shielding placental cells from infection in vivo, we performed bulk and single-cell transcriptomics analyses and found that the maternal-fetal interface of SARS-CoV-2-infected women exhibited robust immune responses, including increased activation of natural killer (NK) and T cells, increased expression of interferon-related genes, as well as markers associated with pregnancy complications such as preeclampsia.
Conclusions: SARS-CoV-2 infection in late pregnancy is associated with immune activation at the maternal-fetal interface even in the absence of detectable local viral invasion.
Funding: NIH (T32GM007205, F30HD093350, K23MH118999, R01AI157488, U01DA040588) and Fast Grant funding support from Emergent Ventures at the Mercatus Center.
Keywords: COVID-19; SARS-CoV-2; placenta; pregnancy.
© 2021 Elsevier Inc.
Conflict of interest statement
A.I. is a scientific advisor for 4BIO and is on the advisory board of Med. The laboratory of A.I. received sponsored research funding from Spring Discovery.
Figures


Update of
-
SARS-CoV-2 infection in pregnancy is associated with robust inflammatory response at the maternal-fetal interface.medRxiv [Preprint]. 2021 Jan 26:2021.01.25.21250452. doi: 10.1101/2021.01.25.21250452. medRxiv. 2021. Update in: Med. 2021 May 14;2(5):591-610.e10. doi: 10.1016/j.medj.2021.04.016. PMID: 33532791 Free PMC article. Updated. Preprint.
References
-
- Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., 3rd, Azziz-Baumgartner E., Gilboa S.M., Meaney-Delman D., CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1641–1647. - PMC - PubMed
-
- Sherer M.L., Lei J., Creisher P., Jang M., Reddy R., Voegtline K., Olson S., Littlefield K., Park H.-S., Ursin R.L., et al. 2020. Dysregulated immunity in SARS-CoV-2 infected pregnant women. medRxiv. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

