Computational structure prediction provides a plausible mechanism for electron transfer by the outer membrane protein Cyc2 from Acidithiobacillus ferrooxidans
- PMID: 33969560
- PMCID: PMC8284587
- DOI: 10.1002/pro.4106
Computational structure prediction provides a plausible mechanism for electron transfer by the outer membrane protein Cyc2 from Acidithiobacillus ferrooxidans
Abstract
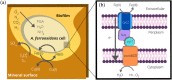
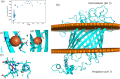
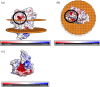
Cyc2 is the key protein in the outer membrane of Acidithiobacillus ferrooxidans that mediates electron transfer between extracellular inorganic iron and the intracellular central metabolism. This cytochrome c is specific for iron and interacts with periplasmic proteins to complete a reversible electron transport chain. A structure of Cyc2 has not yet been characterized experimentally. Here we describe a structural model of Cyc2, and associated proteins, to highlight a plausible mechanism for the ferrous iron electron transfer chain. A comparative modeling protocol specific for trans membrane beta barrel (TMBB) proteins in acidophilic conditions (pH ~ 2) was applied to the primary sequence of Cyc2. The proposed structure has three main regimes: Extracellular loops exposed to low-pH conditions, a TMBB, and an N-terminal cytochrome-like region within the periplasmic space. The Cyc2 model was further refined by identifying likely iron and heme docking sites. This represents the first computational model of Cyc2 that accounts for the membrane microenvironment and the acidity in the extracellular matrix. This approach can be used to model other TMBBs which can be critical for chemolithotrophic microbial growth.
Keywords: Rosetta; chemolithoautotrophy; heme protein; iron oxidation; transmembrane beta barrel.
© 2021 The Protein Society.
Figures

Similar articles
-
Site Directed Mutagenesis of the Cyc2 Outer Membrane Protein from Acidithiobacillus ferrooxidans Reveals a Critical Role for Bound Iron Atoms in Extracellular Electron Transfer.Small. 2025 Mar;21(11):e2408837. doi: 10.1002/smll.202408837. Epub 2025 Feb 12. Small. 2025. PMID: 39937138
-
Increases of ferrous iron oxidation activity and arsenic stressed cell growth by overexpression of Cyc2 in Acidithiobacillus ferrooxidans ATCC19859.Biotechnol Appl Biochem. 2013 Nov-Dec;60(6):623-8. doi: 10.1002/bab.1110. Epub 2013 Aug 23. Biotechnol Appl Biochem. 2013. PMID: 23980744
-
A new iron-oxidizing/O2-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans.J Biol Chem. 2008 Sep 19;283(38):25803-11. doi: 10.1074/jbc.M802496200. Epub 2008 Jul 16. J Biol Chem. 2008. PMID: 18632666 Free PMC article.
-
Mineral respiration under extreme acidic conditions: from a supramolecular organization to a molecular adaptation in Acidithiobacillus ferrooxidans.Biochem Soc Trans. 2012 Dec 1;40(6):1324-9. doi: 10.1042/BST20120141. Biochem Soc Trans. 2012. PMID: 23176476 Review.
-
Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans.World J Microbiol Biotechnol. 2019 Mar 27;35(4):60. doi: 10.1007/s11274-019-2632-y. World J Microbiol Biotechnol. 2019. PMID: 30919119 Review.
Cited by
-
Metagenomic Features Characterized with Microbial Iron Oxidoreduction and Mineral Interaction in Southwest Indian Ridge.Microbiol Spectr. 2022 Dec 21;10(6):e0061422. doi: 10.1128/spectrum.00614-22. Epub 2022 Oct 26. Microbiol Spectr. 2022. PMID: 36286994 Free PMC article.
-
Electrochemical and structural characterization of recombinant respiratory proteins of the acidophilic iron oxidizer Ferrovum sp. PN-J47-F6 suggests adaptations to the acidic pH at protein level.Front Microbiol. 2024 Feb 7;15:1357152. doi: 10.3389/fmicb.2024.1357152. eCollection 2024. Front Microbiol. 2024. PMID: 38384274 Free PMC article.
-
Progress in bioleaching: fundamentals and mechanisms of microbial metal sulfide oxidation - part A.Appl Microbiol Biotechnol. 2022 Nov;106(21):6933-6952. doi: 10.1007/s00253-022-12168-7. Epub 2022 Oct 4. Appl Microbiol Biotechnol. 2022. PMID: 36194263 Free PMC article. Review.
-
Ferric Iron Reduction in Extreme Acidophiles.Front Microbiol. 2022 Jan 12;12:818414. doi: 10.3389/fmicb.2021.818414. eCollection 2021. Front Microbiol. 2022. PMID: 35095822 Free PMC article. Review.
-
Vibrio natriegens as a superior host for the production of c-type cytochromes and difficult-to-express redox proteins.Sci Rep. 2024 Mar 13;14(1):6093. doi: 10.1038/s41598-024-54097-7. Sci Rep. 2024. PMID: 38480761 Free PMC article.
References
-
- Ingledew WJ. Thiobacillus Ferrooxidans the bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta Rev Bioenerg. 1982;683:89–117. - PubMed
-
- Nemati M, Harrison STL, Hansford GS, Webb C. Biological oxidation of ferrous sulphate by Thiobacillus ferrooxidans: a review on the kinetic aspects. Biochem Engin J. 1998;1:171–190.
-
- Priya A, Hait S. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy. 2018;177:132–139.
-
- Li X, Mercado R, Berlinger S, Banta S, West AC. Engineering Acidithiobacillus ferrooxidans growth media for enhanced electrochemical processing. AIChE J. 2014;60:4008–4013.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

