Normalization of cholesterol metabolism in spinal microglia alleviates neuropathic pain
- PMID: 33970188
- PMCID: PMC8111462
- DOI: 10.1084/jem.20202059
Normalization of cholesterol metabolism in spinal microglia alleviates neuropathic pain
Abstract
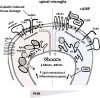
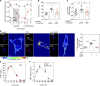
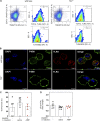
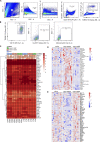
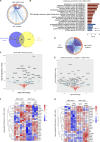
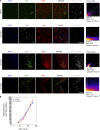
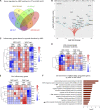
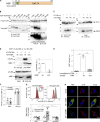
Neuroinflammation is a major component in the transition to and perpetuation of neuropathic pain states. Spinal neuroinflammation involves activation of TLR4, localized to enlarged, cholesterol-enriched lipid rafts, designated here as inflammarafts. Conditional deletion of cholesterol transporters ABCA1 and ABCG1 in microglia, leading to inflammaraft formation, induced tactile allodynia in naive mice. The apoA-I binding protein (AIBP) facilitated cholesterol depletion from inflammarafts and reversed neuropathic pain in a model of chemotherapy-induced peripheral neuropathy (CIPN) in wild-type mice, but AIBP failed to reverse allodynia in mice with ABCA1/ABCG1-deficient microglia, suggesting a cholesterol-dependent mechanism. An AIBP mutant lacking the TLR4-binding domain did not bind microglia or reverse CIPN allodynia. The long-lasting therapeutic effect of a single AIBP dose in CIPN was associated with anti-inflammatory and cholesterol metabolism reprogramming and reduced accumulation of lipid droplets in microglia. These results suggest a cholesterol-driven mechanism of regulation of neuropathic pain by controlling the TLR4 inflammarafts and gene expression program in microglia and blocking the perpetuation of neuroinflammation.
© 2021 Navia-Pelaez et al.
Conflict of interest statement
Disclosures: G. Beaton reported grants from University of California, San Diego, and Epigen Biosciences Inc. during the conduct of the study, and grants from RAFT Pharmaceuticals LLC outside the submitted work. K. Ley reported grants from NIH during the conduct of the study, and grants from Takeda and Novo Nordisk outside the submitted work. M. Corr reported "other" from Gilead outside the submitted work. T.L. Yaksh reported "other" from Raft Pharmaceuticals LLC outside the submitted work; in addition, T.L. Yaksh had a patent to US 10,729,788 B2 issued and is scientific co-founder of Raft Pharmaceuticals LLC. Y.I. Miller reported non-financial support from Raft Pharmaceuticals LLC outside the submitted work; in addition, Y.I. Miller had a patent to US 10,729,788 B2 issued and is scientific co-founder of Raft Pharmaceuticals LLC. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. No other disclosures were reported.
Figures
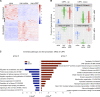
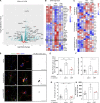

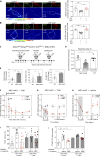
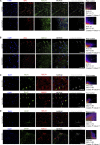



References
-
- Akashi, S., Saitoh S., Wakabayashi Y., Kikuchi T., Takamura N., Nagai Y., Kusumoto Y., Fukase K., Kusumoto S., Adachi Y., et al. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035–1042. 10.1084/jem.20031076 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

