Association of Rotavirus Vaccines With Reduction in Rotavirus Gastroenteritis in Children Younger Than 5 Years: A Systematic Review and Meta-analysis of Randomized Clinical Trials and Observational Studies
- PMID: 33970192
- PMCID: PMC8111566
- DOI: 10.1001/jamapediatrics.2021.0347
Association of Rotavirus Vaccines With Reduction in Rotavirus Gastroenteritis in Children Younger Than 5 Years: A Systematic Review and Meta-analysis of Randomized Clinical Trials and Observational Studies
Abstract
Importance: Rotavirus vaccines have been introduced worldwide, and the clinical association of different rotavirus vaccines with reduction in rotavirus gastroenteritis (RVGE) after introduction are noteworthy.
Objective: To evaluate the comparative benefit, risk, and immunogenicity of different rotavirus vaccines by synthesizing randomized clinical trials (RCTs) and observational studies.
Data sources: Relevant studies published in 4 databases: Embase, PubMed, the Cochrane Library, and Web of Science were searched until July 1, 2020, using search terms including "rotavirus" and "vaccin*."
Study selection: Randomized clinical trials and cohort and case-control studies involving more than 100 children younger than 5 years that reported the effectiveness, safety, or immunogenicity of rotavirus vaccines were included.
Data extraction and synthesis: A random-effects model was used to calculate relative risks (RRs), odds ratios (ORs), risk differences, and 95% CIs. Adjusted indirect treatment comparison was performed to assess the differences in the protection of Rotarix and RotaTeq.
Main outcomes and measures: The primary outcomes were RVGE, severe RVGE, and RVGE hospitalization. Safety-associated outcomes involved serious adverse events, intussusception, and mortality.
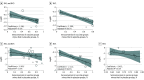
Results: A meta-analysis of 20 RCTs and 38 case-control studies revealed that Rotarix (RV1) significantly reduced RVGE (RR, 0.316 [95% CI, 0.224-0.345]) and RVGE hospitalization risk (OR, 0.347 [95% CI, 0.279-0.432]) among children fully vaccinated; RotaTeq (RV5) had similar outcomes (RVGE: RR, 0.350 [95% CI, 0.275-0.445]; RVGE hospitalization risk: OR, 0.272 [95% CI, 0.197-0.376]). Rotavirus vaccines also demonstrated higher protection against severe RVGE. Additionally, no significant differences in the protection of RV1 and RV5 against rotavirus disease were noted in adjusted indirect comparisons. Moderate associations were found between reduced RVGE risk and Rotavac (RR, 0.664 [95% CI, 0.548-0.804]), Rotasiil (RR, 0.705 [95% CI, 0.605-0.821]), and Lanzhou lamb rotavirus vaccine (RR, 0.407 [95% CI, 0.332-0.499]). All rotavirus vaccines demonstrated no risk of serious adverse events. A positive correlation was also found between immunogenicity and vaccine protection (eg, association of RVGE with RV1: coefficient, -1.599; adjusted R2, 99.7%).
Conclusions and relevance: The high protection and low risk of serious adverse events for rotavirus vaccines in children who were fully vaccinated emphasized the importance of worldwide introduction of rotavirus vaccination. Similar protection provided by Rotarix and RotaTeq relieves the pressure of vaccines selection for health care authorities.
Conflict of interest statement
Figures

Comment in
-
Rotavirus Vaccines-Going Strong After 15 Years.JAMA Pediatr. 2021 Jul 1;175(7):e210356. doi: 10.1001/jamapediatrics.2021.0356. Epub 2021 Jul 6. JAMA Pediatr. 2021. PMID: 33970213 No abstract available.
References
-
- GBD 2016 Diarrhoeal Disease Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211-1228. doi: 10.1016/S1473-3099(18)30362-1 - DOI - PMC - PubMed
-
- Collaborators GDD; GBD Diarrhoeal Diseases Collaborators . Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909-948. doi: 10.1016/S1473-3099(17)30276-1 - DOI - PMC - PubMed
-
- World Health Organization . Rotavirus vaccines: WHO position paper, January 2013. Wkly Epidemiol Rec. 2013;88(5):49-64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

