Role of IL-36γ/IL-36R Signaling in Corneal Innate Defense Against Candida albicans Keratitis
- PMID: 33970198
- PMCID: PMC8114008
- DOI: 10.1167/iovs.62.6.10
Role of IL-36γ/IL-36R Signaling in Corneal Innate Defense Against Candida albicans Keratitis
Abstract
Purpose: Interleukin (IL)-36 cytokines have been shown to play either beneficial or detrimental roles in the infection of mucosal tissues in a pathogen-dependent manner, but their involvement in fungal keratitis remains elusive. We herein investigated their expression and function in mediating corneal innate immunity against Candida albicans infection.
Methods: Gene expression in mouse corneas with or without C. albicans infection was determined by regular RT- and real-time (q)-PCR, Western blot analysis, ELISA or proteome profile assay. The severity of C. albicans keratitis was assessed using clinical scoring, bacterial counting, and myeloperoxidase (MPO) activity as an indicator of neutrophil infiltration. IL36R knockout mice and IL-33-specific siRNA were used to assess the involvement IL-33 signaling in C. albicans-infected corneas. B6 CD11c-DTR mice and clodronate liposomes were used to define the involvement of dendritic cells (DCs) and macrophages in IL-36R signaling and C. albicans keratitis, respectively.
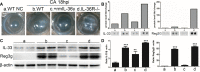
Results: IL-36γ were up-regulated in C57BL6 mouse corneas in response to C. albicans infection. IL-36 receptor-deficient mice display increased severity of keratitis, with a higher fungal load, MPO, and IL-1β levels, and lower soluble sIL-1Ra and calprotectin levels. Exogenous IL-36γ prevented fungal keratitis pathogenesis with lower fungal load and MPO activity, higher expression of sIL-1Ra and calprotectin, and lower expression of IL-1β, at mRNA or protein levels. Protein array analysis revealed that the expression of IL-33 and REG3G were related to IL-36/IL36R signaling, and siRNA downregulation of IL-33 increased the severity of C. albicans keratitis. Depletion of dendritic cells or macrophages resulted in severe C. albicans keratitis and yet exhibited minimal effects on exogenous IL-36γ-induced protection against C. albicans infection in B6 mouse corneas.
Conclusions: IL-36/IL36R signaling plays a protective role in fungal keratitis by promoting AMP expression and by suppressing fungal infection-induced expression of proinflammatory cytokines in a dendritic cell- and macrophage-independent manner.
Conflict of interest statement
Disclosure:
Figures








References
-
- Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004; 15: 321–327. - PubMed
-
- Bhartiya P, Daniell M, Constantinou M, Islam FM, Taylor HR.. Fungal keratitis in Melbourne. Clinical & Experimental Ophthalmology. 2007; 35: 124–130. - PubMed
-
- Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M.. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007; 14: 61–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

