Autosomal recessive osteopetrosis: mechanisms and treatments
- PMID: 33970241
- PMCID: PMC8188884
- DOI: 10.1242/dmm.048940
Autosomal recessive osteopetrosis: mechanisms and treatments
Abstract
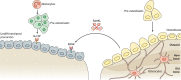
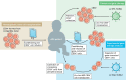
Autosomal recessive osteopetrosis (ARO) is a severe inherited bone disease characterized by defective osteoclast resorption or differentiation. Clinical manifestations include dense and brittle bones, anemia and progressive nerve compression, which hamper the quality of patients' lives and cause death in the first 10 years of age. This Review describes the pathogenesis of ARO and highlights the strengths and weaknesses of the current standard of care, namely hematopoietic stem cell transplantation (HSCT). Despite an improvement in the overall survival and outcomes of HSCT, transplant-related morbidity and the pre-existence of neurological symptoms significantly limit the success of HSCT, while the availability of human leukocyte antigen (HLA)-matched donors still remains an open issue. Novel therapeutic approaches are needed for ARO patients, especially for those that cannot benefit from HSCT. Here, we review preclinical and proof-of-concept studies, such as gene therapy, systematic administration of deficient protein, in utero HSCT and gene editing.
Keywords: Bone disease; Gene therapy; Hematopoietic stem cell transplantation; Osteoclast; Osteopetrosis.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Agarwal, R., Dvorak, C. C., Prohaska, S., Long-Boyle, J., Kwon, H.-S., Brown, J. M., Weinberg, K. I., Le, A., Guttman-Klein, A., Logan, A. C.et al. (2019). Toxicity-free hematopoietic stem cell engraftment achieved with anti-CD117 monoclonal antibody conditioning. Biol. Blood Marrow Transplant. 25 Suppl. 3, S92. 10.1016/j.bbmt.2018.12.172 - DOI
-
- Campeau, P. M., Lu, J. T., Sule, G., Jiang, M. M., Bae, Y., Madan, S., Högler, W., Shaw, N. J., Mumm, S., Gibbs, R. A.et al. (2012). Whole-exome sequencing identifies mutations in the nucleoside transporter gene SLC29A3 in dysosteosclerosis, a form of osteopetrosis. Hum. Mol. Genet. 21, 4904-4909. 10.1093/hmg/dds326 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

