Patient-Reported Outcomes of Treatment of Opioid Dependence With Weekly and Monthly Subcutaneous Depot vs Daily Sublingual Buprenorphine: A Randomized Clinical Trial
- PMID: 33970256
- PMCID: PMC8111483
- DOI: 10.1001/jamanetworkopen.2021.9041
Patient-Reported Outcomes of Treatment of Opioid Dependence With Weekly and Monthly Subcutaneous Depot vs Daily Sublingual Buprenorphine: A Randomized Clinical Trial
Abstract
Importance: Patient-reported outcomes in the treatment of opioid dependence may differ between subcutaneously administered depot buprenorphine and daily sublingual buprenorphine.
Objective: To compare patient satisfaction between depot buprenorphine and sublingual buprenorphine in adult outpatients with opioid dependence.
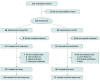
Design, setting, and participants: This open-label, randomized clinical trial was conducted among adult patients with opioid dependence at 6 outpatient clinical sites in Australia from October 2018 to September 2019. Data analysis was conducted from October 2019 to May 2020.
Interventions: Participants were randomized to receive treatment with weekly or monthly depot buprenorphine or daily sublingual buprenorphine over 24 weeks.
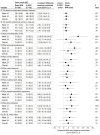
Main outcomes and measures: The primary end point was the difference in global treatment satisfaction, assessed by the Treatment Satisfaction Questionnaire for Medication (TSQM) version 1.4 (range, 0-100; higher score indicates greater satisfaction) at week 24. Secondary end points included other patient-reported outcomes, including quality of life, treatment burden, and health-related outcomes, as well as measures of opioid use, retention in treatment, and safety.
Results: A total of 119 participants (70 [58.8%] men; mean [SD] age, 44.4 [10.5] years) were enrolled, randomized to, and received either depot buprenorphine (60 participants [50.4%]) or sublingual buprenorphine (59 participants [49.6%]). From the initial sample of 120, a participant (0.8%) in the sublingual buprenorphine group withdrew consent and did not receive study treatment. All participants were receiving sublingual buprenorphine when enrolled. The mean TSQM global satisfaction score was significantly higher for the depot group compared with the sublingual group at week 24 (mean [SE] score, 82.5 [2.3] vs 74.3 [2.3]; difference, 8.2; 95% CI, 1.7 to 14.6; P = .01). Improved outcomes were also observed for several secondary end points after treatment with depot buprenorphine (eg, mean [SE] treatment burden assessed by the Treatment Burden Questionnaire global score, on which lower scores indicate lower burden: 13.2 [2.6] vs 28.6 [2.5]; difference, -15.4; 95% CI, -22.6 to -8.2; P < .001). Thirty-nine participants (65.0%) in the depot buprenorphine group experienced 117 adverse drug reactions, mainly injection site reactions of mild intensity following subcutaneous administration, and 12 participants (20.3%) in the sublingual buprenorphine group experienced 21 adverse drug reactions. No participants withdrew from the trial medication or the trial due to adverse events.
Conclusions and relevance: In this study, participants receiving depot buprenorphine reported improved treatment satisfaction compared with those receiving sublingual buprenorphine. The results highlight the application of patient-reported outcomes as alternative end points to traditional markers of substance use in addiction treatment outcome studies.
Trial registration: anzctr.org.au Identifier: ANZCTR12618001759280.
Conflict of interest statement
Figures

Comment in
-
Extended-Release Buprenorphine and Its Evaluation With Patient-Reported Outcomes.JAMA Netw Open. 2021 May 3;4(5):e219708. doi: 10.1001/jamanetworkopen.2021.9708. JAMA Netw Open. 2021. PMID: 33970262 No abstract available.
References
-
- World Health Organization . World Health Organization model list of essential medicines: 21st list 2019. Published 2019. Accessed April 1, 2021. https://apps.who.int/iris/handle/10665/325771
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

