Sustaining Meaningful Patient Engagement Across the Lifecycle of Medicines: A Roadmap for Action
- PMID: 33970465
- PMCID: PMC8108434
- DOI: 10.1007/s43441-021-00282-z
Sustaining Meaningful Patient Engagement Across the Lifecycle of Medicines: A Roadmap for Action
Abstract
Background: There is increased recognition that incorporating patients' perspectives and insights into the medicines development process results in better health outcomes and benefits for all involved stakeholders. Despite the increased interest and the existence of frameworks and practical recommendations, patient engagement (PE) is not yet considered standard practice. The objective of this work was to provide a roadmap to support systematic change in all stakeholder organisations involved in medicines development across Europe, patients and patient organisations, medicines developers, academia, regulatory authorities, Health Technology Assessment bodies, payers, policy-makers and public research funders, to sustain PE practices.
Methods: A mixed-methods approach was used by the EU-funded Innovative Medicines Initiative PARADIGM Consortium to co-develop the sustainability roadmap including background work to identify success factors and scenarios for sustainable PE. The roadmap development was based on the Theory of Change concept and populated with findings from (1) interviews with national/ and international institutions with the potential to increase PE uptake by other stakeholders; (2) multi-stakeholder workshops and webinars; and (3) consultations with specific stakeholder groups, Consortium members and a consultative body formed by international PE initiatives.
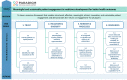
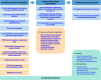
Results: This roadmap sets strategic goals for the PE community to achieve meaningful and systematic PE through changes in the culture, processes and resources of stakeholder organisations. It brings in key PARADIGM outputs to work in a coordinated fashion with existing frameworks and mechanisms to achieve system-wide sustained PE.
Conclusions: The roadmap provides a framework for all stakeholders to take collective action within their organisations and across Europe to implement PE in a sustainable manner.
Keywords: Call to action; Medicines development; Patient engagement; Roadmap; Sustainability.
© 2021. The Author(s).
Conflict of interest statement
The following authors have no conflict of interest to disclose: Maria Cavaller Bellaubi, Stuart D. Faulkner, Bryan Teixeira, Mathieu Boudes, Eva Molero, Nicholas Brooke, Laura McKeaveney, Jeffrey Southerton, Maria José Vicente, Juan García-Burgos, Vinciane Pirard, Kirsty Reid and Elisa Ferrer. Neil Bertelsen works with a variety of stakeholder groups on patient involvement in healthcare decisions. This includes work as a consultant to patient groups and to the industry.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

