Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome
- PMID: 33970483
- PMCID: PMC8109227
- DOI: 10.1002/14651858.CD011672.pub2
Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome
Abstract
Background: Non-invasive respiratory support is increasingly used for the management of respiratory dysfunction in preterm infants. This approach runs the risk of under-treating those with respiratory distress syndrome (RDS), for whom surfactant administration is of paramount importance. Several techniques of minimally invasive surfactant therapy have been described. This review focuses on surfactant administration to spontaneously breathing infants via a thin catheter briefly inserted into the trachea.
Objectives: Primary objectives In non-intubated preterm infants with established RDS or at risk of developing RDS to compare surfactant administration via thin catheter with: 1. intubation and surfactant administration through an endotracheal tube (ETT); or 2. continuation of non-invasive respiratory support without surfactant administration or intubation. Secondary objective 1. To compare different methods of surfactant administration via thin catheter Planned subgroup analyses included gestational age, timing of intervention, and use of sedating pre-medication during the intervention.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions(R); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), on 30 September 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi-randomised trials.
Selection criteria: We included randomised trials comparing surfactant administration via thin catheter (S-TC) with (1) surfactant administration through an ETT (S-ETT), or (2) continuation of non-invasive respiratory support without surfactant administration or intubation. We also included trials comparing different methods/strategies of surfactant administration via thin catheter. We included preterm infants (at < 37 weeks' gestation) with or at risk of RDS.
Data collection and analysis: Review authors independently assessed study quality and risk of bias and extracted data. Authors of all studies were contacted regarding study design and/or missing or unpublished data. We used the GRADE approach to assess the certainty of evidence.
Main results: We included 16 studies (18 publications; 2164 neonates) in this review. These studies compared surfactant administration via thin catheter with surfactant administration through an ETT with early extubation (Intubate, Surfactant, Extubate technique - InSurE) (12 studies) or with delayed extubation (2 studies), or with continuation of continuous positive airway pressure (CPAP) and rescue surfactant administration at pre-specified criteria (1 study), or compared different strategies of surfactant administration via thin catheter (1 study). Two trials reported neurosensory outcomes of of surviving participants at two years of age. Eight studies were of moderate certainty with low risk of bias, and eight studies were of lower certainty with unclear risk of bias. S-TC versus S-ETT in preterm infants with or at risk of RDS Meta-analyses of 14 studies in which S-TC was compared with S-ETT as a control demonstrated a significant decrease in risk of the composite outcome of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age (risk ratio (RR) 0.59, 95% confidence interval (CI) 0.48 to 0.73; risk difference (RD) -0.11, 95% CI -0.15 to -0.07; number needed to treat for an additional beneficial outcome (NNTB) 9, 95% CI 7 to 16; 10 studies; 1324 infants; moderate-certainty evidence); the need for intubation within 72 hours (RR 0.63, 95% CI 0.54 to 0.74; RD -0.14, 95% CI -0.18 to -0.09; NNTB 8, 95% CI; 6 to 12; 12 studies, 1422 infants; moderate-certainty evidence); severe intraventricular haemorrhage (RR 0.63, 95% CI 0.42 to 0.96; RD -0.04, 95% CI -0.08 to -0.00; NNTB 22, 95% CI 12 to 193; 5 studies, 857 infants; low-certainty evidence); death during first hospitalisation (RR 0.63, 95% CI 0.47 to 0.84; RD -0.02, 95% CI -0.10 to 0.06; NNTB 20, 95% CI 12 to 58; 11 studies, 1424 infants; low-certainty evidence); and BPD among survivors (RR 0.57, 95% CI 0.45 to 0.74; RD -0.08, 95% CI -0.11 to -0.04; NNTB 13, 95% CI 9 to 24; 11 studies, 1567 infants; moderate-certainty evidence). There was no significant difference in risk of air leak requiring drainage (RR 0.58, 95% CI 0.33 to 1.02; RD -0.03, 95% CI -0.05 to 0.00; 6 studies, 1036 infants; low-certainty evidence). None of the studies reported on the outcome of death or survival with neurosensory disability. Only one trial compared surfactant delivery via thin catheter with continuation of CPAP, and one trial compared different strategies of surfactant delivery via thin catheter, precluding meta-analysis.
Authors' conclusions: Administration of surfactant via thin catheter compared with administration via an ETT is associated with reduced risk of death or BPD, less intubation in the first 72 hours, and reduced incidence of major complications and in-hospital mortality. This procedure had a similar rate of adverse effects as surfactant administration through an ETT. Data suggest that treatment with surfactant via thin catheter may be preferable to surfactant therapy by ETT. Further well-designed studies of adequate size and power, as well as ongoing studies, will help confirm and refine these findings, clarify whether surfactant therapy via thin tracheal catheter provides benefits over continuation of non-invasive respiratory support without surfactant, address uncertainties within important subgroups, and clarify the role of sedation.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MEA has no interests to declare.
KIW has no interests to declare.
PGD has no interests to declare.
AGDP has no interests to declare.
PAD is the Chief Investigator of the OPTIMIST‐A trial, a multi‐centre RCT of surfactant via tracheal catheterisation in preterm infants on CPAP (
Figures
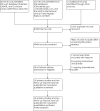
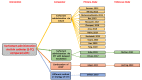

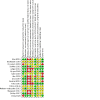

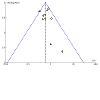


























































Update of
References
References to studies included in this review
Bao 2015 {published and unpublished data}
Boskabadi 2019 {published data only}
-
- Boskabadi H, Maamouri G, Gharaei Jomeh R, Zakerihamidi M. Comparative study of the effect of the administration of surfactant through a thin endotracheal catheter into trachea during spontaneous breathing with intubation (intubation-surfactant-extubation method). Journal of Clinical Neonatology 2019;8(4):227-31. [DOI: 10.4103/jcn.JCN_32_19] - DOI
Choupani 2018 {published data only}
-
- Choupani R, Mashayekhy G, Hmidi M, Kheiri S, Khalili Dehkordi M. A comparative study of the efficacy of surfactant administration through a thin intratracheal catheter and its administration via an endotracheal tube in neonatal respiratory distress syndrome. Iranian Journal of Neonatology 2018;9(4):33-40. [DOI: 10.22038/ijn.2018.30057.1408] - DOI
Dekker 2019 {published and unpublished data}
-
- Dekker J, Lopriore E, Zanten HA, Tan RN, Hooper SB, te Pas AB. Sedation during minimal invasive surfactant therapy: a randomised controlled trial (PROMISES). Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(4):F378–83. [DOI: 10.1136/archdischild-2018-315015] [PMID: ] - DOI - PubMed
Göpel 2011 {published data only}
-
- Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al, German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants: an open-label, randomised, controlled trial (AMV Trial). Lancet 2011;378(9803):1727-34. [DOI: 10.1016/S0140-6736(11)60986-0] [PMID: ] - DOI - PubMed
-
- Herting E, Kribs A, Härtel C, Wense A, Weller U, Hoehn T, et al, German Neonatal Network (GNN). Two-year outcome data suggest that less invasive surfactant administration (LISA) is safe. Results from the follow-up of the randomized controlled AMV (avoid mechanical ventilation) study. European Journal of Pediatrics 2020;179(8):1309-13. [DOI: 10.1007/s00431-020-03572-0] [PMID: ] - DOI - PMC - PubMed
Gupta 2020 {published data only}
-
- Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation - a randomized controlled trial. European Journal of Pediatrics 2020;179(8):1287–93. [DOI: 10.1007/s00431-020-03682-9] [PMID: ] - DOI - PMC - PubMed
Halim 2019 {published data only}
Han 2020 {published and unpublished data}
-
- Han T, Liu H, Zhang H, Guo M, Zhang X, Duan Y, et al. Minimally invasive surfactant administration for the treatment of neonatal respiratory distress syndrome: a multicenter randomized study in China. Frontiers in Pediatrics 2020;8(182):1-12. [DOI: 10.3389/fped.2020.00182] [PMID: ] - DOI - PMC - PubMed
Jena 2019 {published and unpublished data}
-
- Jena SR, Bains HS, Pandita A, Verma A, Gupta V, Kallem VR, et al, On Behalf of Sure Group. Surfactant therapy in premature babies: SurE or InSurE. Pediatric Pulmonology 2019;54(11):1747-52. [DOI: ] [PMID: ] - PubMed
Kanmaz 2013 {published and unpublished data}
Kribs 2015 {published data only}
-
- Kribs A, Roll C, Göpel W, Wieg C, Groneck P, Laux R, Teig N, et al, NINSAPP Trial Investigators. Nonintubated surfactant application vs conventional therapy in extremely preterm infants a randomized clinical trial. JAMA Pediatrics 2015;169(8):723-30. [DOI: 10.1001/jamapediatrics.2015.0504] [PMID: ] - DOI - PubMed
Mirnia 2013a {published data only}
-
- Mirnia K, Heidarzadeh M, Hosseini MB, Sadeghnia A, Balila M, Ghojazadeh M. Comparison outcome of surfactant administration via tracheal catheterization during spontaneous breathing with INSURE. Medical Journal of Islamic World Academy of Sciences 2013;21(4):143-8. [DOI: 10.12816/0002647] - DOI
Mohammadizadeh 2015 {published data only}
-
- Mohammadizadeh M, Ardestani AG, Sadeghnia AR. Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. Journal of Research in Pharmacy Practice 2015;4(1):31-6. [DOI: 10.4103/2279-042X.150053] [PMID: ] - DOI - PMC - PubMed
Mosayebi 2017 {published data only}
-
- Mosayebi Z, Kadivar M, Taheri-Derakhsh N, Nariman S, Mahdi Marash Si, Farsi Z. A randomized trial comparing surfactant administration using InSurE technique and the minimally invasive surfactant therapy in preterm infants (28 to 34 weeks of gestation) with respiratory distress syndrome. Journal of Comprehensive Pediatrics 2017;8(4):e60724. [DOI: 10.5812/compreped.60724] - DOI
Olivier 2017 {published and unpublished data}
Yang 2020 {published and unpublished data}
-
- Yang G, Hei M, Xue Z, Zhao Y, Zhang X, Wang C. Effects of less invasive surfactant administration (LISA) via a gastric tube on the treatment of respiratory distress syndrome in premature infants aged 32 to 36 weeks. Medicine (Baltimore) 2020;99(9):e19216. [DOI: 10.1097/MD.0000000000019216] [PMID: ] - DOI - PMC - PubMed
References to studies excluded from this review
Mirnia 2013b {published data only}
-
- Heidarzadeh M, Mirnia K, Hoseini MB, Sadeghnia A, Akrami F, Balila M, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial in Alzahra Hospital. Iranian Journal of Neonatology 2013;4(2):5-9. [DOI: 10.22038/IJN.2013.1075] [ijn.mums.ac.ir/article_1075.html] - DOI
-
- Mirnia K, Heidarzadeh M, Hoseini MB, Sadeghnia A, Akrami F, Balila M, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial in Alzahra Hospital. Iranian Journal of Neonatology 2013;4(2):5-9. [DOI: 10.22038/IJN.2013.1075] [jn.mums.ac.ir/article_1075.html] - DOI
Oncel 2016 {published data only}
-
- Oncel MY, Arayici S, Uras N, Alyamac-Dizdar E, Sari FN, Karahan S, et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Archives of Disease in Childhood. Fetal Neonatal Edition 2016;101(4):F323-8. [DOI: 10.1136/archdischild-2015-308204] [PMID: ] - DOI - PubMed
References to ongoing studies
ACTRN12611000916943 {published data only}
-
- ACTRN12611000916943. OPTIMIST-A trial: multicentre randomised controlled trial in preterm infants 25-28 weeks gestation on continuous positive airway pressure of the effect of minimally-invasive surfactant therapy in comparison to standard care (continuation of CPAP) on the incidence of the composite outcome of death or physiological BPD. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336668 (first received 25 August 2011). [https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336668&... (Registered on 26 Aug 2011)]
-
- NCT02140580. OPTIMIST-A trial: minimally-invasive surfactant therapy in preterm infants 25-28 weeks gestation on CPAP (OPTIMIST-A) [Multicentre randomised controlled trial of minimally-invasive surfactant therapy in preterm infants 25-28 weeks gestation on continuous positive airways pressure]. clinicaltrials.gov/ct2/show/NCT02140580 (first received 16 May 2014).
ACTRN12611000917932 {published data only}
-
- ACTRN12611000917932. The OPTIMIST-B trial: multicentre randomised controlled trial in preterm infants 29-32 weeks gestation on continuous positive airway pressure of the effect of minimally-invasive surfactant therapy in comparison to standard care (continuation of CPAP) on the duration of respiratory support (all hours of intubation, nasal CPAP and high flow nasal cannula). anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343305 (first received 26 August 2011).
ChiCTR1900020970 {published data only}
-
- ChiCTR1900020970. A multicenter clinical randomized controlled study comparing the application of modified minimally invasive pulmonary surfactant and low inspiratory peak pressure supporting pulmonary surfactant instillation technology in the treatment of respiratory distress syndrome in very premature infants. chictr.org.cn/searchproj.aspx?ishtml=sponsorproj&type=cn&institu... (first received 23 January 2019). [DOI: 10.1186/s13063-020-04390-3] - DOI
NCT01615016 {published data only}
-
- NCT01615016. MISurf versus InSurE. A comparison of minimally invasive surfactant application techniques in preterm infants (MIsurf) [Feasibility study of a comparison of minimally invasive surfactant application techniques in preterm infants]. clinicaltrials.gov/ct2/show/NCT01615016 (first received 8 June 2012).
NCT01848262 {published data only}
-
- NCT01848262. ECALMIST versus InSurE in preterm infant < 32 weeks, multicenter, multinational RCT (ECALMIST) [ECALMIST (Early CPAP And Large Volume Minimal Invasive Surfactant Therapy) versus InSurE (Intubate, Surfactant, Extubate) in preterm infants with respiratory distress syndrome (RDS): prospective randomised control clinical trial]. clinicaltrials.gov/ct2/show/NCT01848262 (first received 7 May 2013).
NCT02772081 {published data only}
-
- NCT02772081. An open-label, multicenter, randomized, controlled study in spontaneously breathing preterm neonates with respiratory distress syndrome to compare two procedures for porcine surfactant (poractant alfa, CUROSURF®) administration: a less invasive method (LISA) during non-invasive ventilation (NIV) and the conventional administration during brief invasive ventilation (LISPAP). ClinicalTrials.gov/show/NCT02772081 (first received 13 May 2019).
NCT03989960 {published data only}
-
- NCT03989960. Application of modified intubation-surfactant-extubation (InSurE) technique in preterm neonates with respiratory distress syndrome (MOLISAN). ClinicalTrials.gov/show/NCT03989960 (first received 18 June 2019).
NCT04016246 {published data only}
-
- NCT04016246 Chevallier M, Durrmeyer X, Ego A, Debillon T, the PROLISA Study Group. Propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: study protocol for a multicenter, double-blind, placebo controlled trial (PROLISA). BMC Pediatrics 2020;20(100):1-9. [DOI: 10.1186/s12887-020-02112-x] - DOI - PMC - PubMed
-
- NCT04016246. Respiratory effect of the LISA (less invasive surfactant administration) method with sedation by propofol versus absence of sedation: double-blind comparative randomized clinical trial (PROLISA). ClinicalTrials.gov/show/NCT04016246 (first received 11 July 2019).
NCT04073173 {published data only}
-
- NCT04073173. Stress assessment in preterm infants with respiratory distress syndrome treated or not with an analgesic drug during the traditional or the less invasive method of surfactant therapy (StrAAS). clinicaltrials.gov/ct2/show/NCT04073173 (first received 29 August 2020).
NCT04445571 {published data only}
-
- NCT04445571. Surfactant Administration by Insure or Thin Catheter (SAINT). clinicaltrials.gov/ct2/show/NCT0444557 (first received 24 June 2020).
UMIN000021785 {published data only}
-
- UMIN000021785. Effectiveness of MIST (minimally invasive surfactant therapy) under bronchoscopy in treating neonatal respiratory distress syndrome. rctportal.niph.go.jp/en/detail?trial_id=UMIN000021785 (first received 5 April 2016).
Additional references
Abdel‐Latif 2011a
-
- Abdel-Latif ME, Osborn DA. Pharyngeal instillation of surfactant before the first breath for prevention of morbidity and mortality in preterm infants at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD008311. [DOI: 10.1002/14651858.CD008311.pub2] - DOI - PubMed
Abdel‐Latif 2011b
-
- Abdel-Latif ME, Osborn D. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008309. [DOI: 10.1002/14651858.CD008309.pub2] - DOI - PubMed
Abdel‐Latif 2012
Aguar 2014
-
- Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy (MIST) with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr 2014;103(6):e229-33 2014;103(6):e229-33. [DOI: 10.1111/apa.12611] [PMID: ] - DOI - PubMed
Aldana‐Aguirre 2016
-
- Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Archieves of Disease in Childhood. Fetal and Neonatalal Edition 2016;102(1):F17-23. [DOI: 10.1136/archdischild-2015-310299] [PMID: ] - DOI - PubMed
Ammari 2005
Avery 1959
Bell 1978
Berger 2013
Bhayat 2020
Björklund 1997
-
- Björklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatric Research 1997;42(3):348-55. [DOI: 10.1203/00006450-199709000-00016] [PMID: ] - DOI - PubMed
Dargaville 2011
Dargaville 2013a
-
- Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Archives of Disease in Childhood. Fetal Neonatal Edition 2013;98(2):F122-6. [DOI: 10.1136/archdischild-2011-301314] [PMID: ] - DOI - PubMed
Dargaville 2013b
Dargaville 2016
Dekker 2016
Dunn 2011
Egger 1997
Finer 2010
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 2 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gupta 2012
Haberman 2002
-
- Haberman B, Shankaran S, Stevenson DK, Papile LA, Stark A, Korones S, et al. Does surfactant and immediate extubation to nasal continuous positive airway pressure (CPAP) reduce use of mechanical ventilation? Pediatric Research 2002;51(4 Suppl):349A.
Heiring 2017
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Isayama 2016
Jeffreys 2019
Jobe 1993
Klotz 2017
Kribs 2007
-
- Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤ 27 weeks). Paediatric Anaesthesia 2007;17(4):264-9. [DOI: ] [PMID: ] - PubMed
Kribs 2008
Kribs 2009
Kribs 2010
Lau 2017
Mehler 2013
More 2014
Morley 2008
Panza 2020
-
- Panza R, Laforgia N, Bellos I, Pandita A. Systematic review found that using thin catheters to deliver surfactant to preterm neonates was associated with reduced bronchopulmonary dysplasia and mechanical ventilation. Acta Paediatrica 2020;109(11):2219-2225. [DOI: 10.1111/apa.15374] [PMID: ] - DOI - PubMed
Papile 1978
Porth 2011
Reininger 2005
-
- Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, et al. Surfactant administration by transient intubation in infants 29 to 35 weeks' gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: a randomized controlled trial. Journal of Perinatology 2005;25(11):703-8. [DOI: 10.1038/sj.jp.7211381] [PMID: ] - DOI - PubMed
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roberts 2020
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Shennan 1988
-
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527-32. [PMID: ] - PubMed
Sinclair 2009
-
- Sinclair SE, Chi E, Lin HI, Altemeier WA. Positive end-expiratory pressure alters the severity and spatial heterogeneity of ventilator-induced lung injury: an argument for cyclical airway collapse. Journal of Critical Care 2009;24(2):206-11. [DOI: 10.1016/j.jcrc.2008.04.005] [PMID: ] - DOI - PMC - PubMed
Soll 2013
Stevens 2007
-
- Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD003063. [DOI: 10.1002/14651858.CD003063.pub3] - DOI - PMC - PubMed
Suresh 2005
Sweet 2016
Verder 1994
-
- Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. New England Journal of Medicine 1994;331(16):1051-5. [DOI: 10.1056/NEJM199410203311603] [PMID: ] - DOI - PubMed
Victorin 1990
Walsh 1988
Walsh 2004
-
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] - DOI - PubMed
Wu 2017
Zwicker 2016
References to other published versions of this review
Wheeler 2015
-
- Wheeler KI, Abdel‐Latif ME, Davis PG, De Paoli AG, Dargaville PA. Surfactant therapy via brief tracheal catheterization in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD011672. [DOI: 10.1002/14651858.CD011672] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

