Magnesium depletion extends fission yeast lifespan via general amino acid control activation
- PMID: 33970532
- PMCID: PMC8088111
- DOI: 10.1002/mbo3.1176
Magnesium depletion extends fission yeast lifespan via general amino acid control activation
Abstract
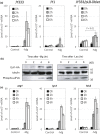
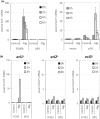
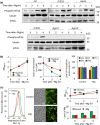
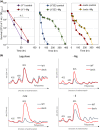
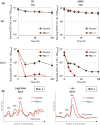
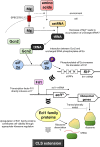
Nutrients including glucose, nitrogen, sulfur, zinc, and iron are involved in the regulation of chronological lifespan (CLS) of yeast, which serves as a model of the lifespan of differentiated cells of higher organisms. Herein, we show that magnesium (Mg2+ ) depletion extends CLS of the fission yeast Schizosaccharomyces pombe through a mechanism involving the Ecl1 gene family. We discovered that ecl1+ expression, which extends CLS, responds to Mg2+ depletion. Therefore, we investigated the underlying intracellular responses. In amino acid auxotrophic strains, Mg2+ depletion robustly induces ecl1+ expression through the activation of the general amino acid control (GAAC) pathway-the equivalent of the amino acid response of mammals. Polysome analysis indicated that the expression of Ecl1 family genes was required for regulating ribosome amount when cells were starved, suggesting that Ecl1 family gene products control the abundance of ribosomes, which contributes to longevity through the activation of the evolutionarily conserved GAAC pathway. The present study extends our understanding of the cellular response to Mg2+ depletion and its influence on the mechanism controlling longevity.
Keywords: Ecl1 family gene; GAAC; chronological lifespan; fission yeast; magnesium.
© 2021 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Airas, R. K. (2007). Magnesium dependence of the measured equilibrium constants of aminoacyl‐tRNA synthetases. Biophysical Chemistry, 131, 29–35. - PubMed
-
- Akanuma, G., Kazo, Y., Tagami, K., Hiraoka, H., Yano, K., Suzuki, S., Hanai, R., Nanamiya, H., Kato‐Yamada, Y., & Kawamura, F. (2016). Ribosome dimerization is essential for the efficient regrowth of Bacillus subtilis . Microbiology, 162, 448–458. - PubMed
-
- Akanuma, G., Nanamiya, H., Natori, Y., Yano, K., Suzuki, S., Omata, S., Ishizuka, M., Sekine, Y., & Kawamura, F. (2012). Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. Journal of Bacteriology, 194, 6282–6291. - PMC - PubMed
-
- Akanuma, G., Yamazaki, K., Yagishi, Y., Iizuka, Y., Ishizuka, M., Kawamura, F., & Kato‐Yamada, Y. (2018). Magnesium suppresses defects in the formation of 70S ribosomes as well as in sporulation caused by lack of several individual ribosomal proteins. Journal of Bacteriology, 200, 1–11. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

