Assessing the Pregnancy Protective Impact of Scheduled Nonadherence to a Novel Progestin-Only Pill: Protocol for a Prospective, Multicenter, Randomized, Crossover Study
- PMID: 33970869
- PMCID: PMC8262664
- DOI: 10.2196/29208
Assessing the Pregnancy Protective Impact of Scheduled Nonadherence to a Novel Progestin-Only Pill: Protocol for a Prospective, Multicenter, Randomized, Crossover Study
Abstract
Background: Progestin-only contraceptive pills (POP) are commonly reserved for women with medical comorbidities but in actuality, POPs can be safely used by anyone wanting to prevent pregnancy. This wide safety profile makes them an ideal candidate for being available over the counter without a prescription, but adherence issues may be more common with over-the-counter use. We need a better understanding of the ability of POPs to prevent pregnancy when adherence issues occur in the form of a missed or delayed pill.
Objective: This study aims to determine cervical mucus characteristics following a 6-hour delayed pill intake or after one missed pill as compared to typical daily use of norgestrel 75 mcg.
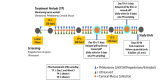
Methods: This prospective, multicenter, randomized, crossover study assesses the effect of norgestrel 75 mcg (Opill) on cervical mucus and ovarian activity during reported compliant daily use, after a 6-hour delayed intake mid cycle, and after a mid-cycle missed pill. Subject participation will last approximately 4.5 months. We will recruit at 2 US sites: Oregon Health & Science University, Portland, Oregon and University of California Davis Health, Sacramento, California. Reproductive-aged subjects with regular menstrual cycles (21-35 days), BMI <32 kg/m2, and proven ovulation (screening luteal phase progesterone >3 ng/mL [>10 nmol/L]) are eligible to enroll. Participants cannot be at risk for pregnancy during the study period and not use other hormonal methods. Norgestrel 75 mcg will be taken at the same time daily except for one day in each of treatment periods 2 and 3, when the pill will be taken either 6 hours late (delayed pill) or omitted completely (missed pill). Every 3-4 days, we will monitor subjects for follicular activity with transvaginal ultrasound (TVUS) examination, cervical mucus, and blood sampling for ovarian hormones and gonadotropins. Subjects will undergo serial cervical mucus sampling on the days with missed and delayed pill intake at 8 hours after pill intake on the day before the delayed or missed pill, 3 hours following the scheduled time of pill intake if intake was delayed, 6 hours after the scheduled time if intake was omitted, and on the next day 30 minutes before the time of scheduled pill intake. The primary objective of the study is to determine the effect of a delayed or omitted pill intake on cervical mucus characteristics based on a modified Insler score compared to reported daily use.
Results: Our protocol was successfully approved by a central institutional review board (Advarra, Columbia, MD), received ethical approval on March 23, 2018, and was registered with ClinicalTrials.gov (NCT03585712). As of January 2020, the study completed enrollment of 52 subjects. Analyses are pending.
Conclusions: Our protocol was approved by a central review board, and study procedures were successfully executed with completed proposed enrollment.
Trial registration: ClinicalTrials.gov NCT03585712; https://clinicaltrials.gov/ct2/show/NCT03585712.
International registered report identifier (irrid): DERR1-10.2196/29208.
Keywords: contraception; missed pill; pharmacokinetics; progestin-only pills; protocol.
©Alison Edelman, Agnes Hemon, Mitchell Creinin, Pascale Borensztein, Bruno Scherrer, Anna Glasier. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 08.06.2021.
Conflict of interest statement
Conflicts of Interest: AE reports honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. AE receives royalties from Up to Date, Inc, and she has acted as a consultant to MedinCELL. Oregon Health & Science University receives research funding from Oregon Health & Science University Foundation, Merck, HRA Pharma, and National Institutes of Health where AE is the principal investigator. AH and PB are employees of HRA Pharma. MC serves on an Advisory Board for Evofem, Mayne, Merck, and Searchlight and is a consultant for Danco, Estetra, Mayne, Medicines360, and Merck. The Department of Obstetrics and Gynecology, University of California, Davis Health receives contraceptive research funding, from which MC is supported from HRA Pharma, Medicines360, Merck, and Sebela. BS and AG are regular consultants to HRA Pharma.
Figures
Similar articles
-
The effect of deliberate non-adherence to a norgestrel progestin-only pill: A randomized, crossover study.Contraception. 2023 Jan;117:1-6. doi: 10.1016/j.contraception.2022.09.002. Epub 2022 Sep 18. Contraception. 2023. PMID: 36130667 Clinical Trial.
-
Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception.Contraception. 2022 Aug;112:43-47. doi: 10.1016/j.contraception.2022.03.016. Epub 2022 Mar 31. Contraception. 2022. PMID: 35367204
-
Maintenance of ovulation inhibition with a new progestogen-only pill containing drospirenone after scheduled 24-h delays in pill intake.Contraception. 2016 Apr;93(4):303-309. doi: 10.1016/j.contraception.2015.12.007. Epub 2015 Dec 17. Contraception. 2016. PMID: 26708301 Clinical Trial.
-
The progestogen-only mini-pill.Contraception. 1982 Oct;26(4):373-88. doi: 10.1016/0010-7824(82)90104-4. Contraception. 1982. PMID: 6759029 Review.
-
Why do inadvertent pregnancies occur in oral contraceptive users? Effectiveness of oral contraceptive regimens and interfering factors.Contraception. 1983 Jun;27(6):531-51. doi: 10.1016/0010-7824(83)90019-7. Contraception. 1983. PMID: 6413129 Review.
References
-
- Kavanaugh ML, Pliskin E, Jerman J. Use of concurrent multiple methods of contraception in the United States, 2008 to 2015. Contracept X. 2021;3:100060. doi: 10.1016/j.conx.2021.100060. https://linkinghub.elsevier.com/retrieve/pii/S2590-1516(21)00007-1 - DOI - PMC - PubMed
-
- US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. Centers for Disease Control and Prevention. 2016. [2021-05-18]. https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html.
-
- White K, Potter JE, Hopkins K, Fernández L, Amastae J, Grossman D. Contraindications to progestin-only oral contraceptive pills among reproductive-aged women. Contraception. 2012 Sep;86(3):199–203. doi: 10.1016/j.contraception.2012.01.008. http://europepmc.org/abstract/MED/22364816 - DOI - PMC - PubMed
-
- Grossman D, Fernandez L, Hopkins K, Amastae J, Garcia SG, Potter JE. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008 Sep;112(3):572–8. doi: 10.1097/AOG.0b013e31818345f0. http://europepmc.org/abstract/MED/18757654 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials