Agent-based model provides insight into the mechanisms behind failed regeneration following volumetric muscle loss injury
- PMID: 33970905
- PMCID: PMC8110270
- DOI: 10.1371/journal.pcbi.1008937
Agent-based model provides insight into the mechanisms behind failed regeneration following volumetric muscle loss injury
Abstract
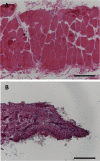
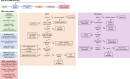
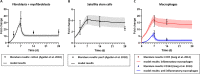
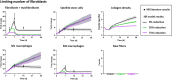
Skeletal muscle possesses a remarkable capacity for repair and regeneration following a variety of injuries. When successful, this highly orchestrated regenerative process requires the contribution of several muscle resident cell populations including satellite stem cells (SSCs), fibroblasts, macrophages and vascular cells. However, volumetric muscle loss injuries (VML) involve simultaneous destruction of multiple tissue components (e.g., as a result of battlefield injuries or vehicular accidents) and are so extensive that they exceed the intrinsic capability for scarless wound healing and result in permanent cosmetic and functional deficits. In this scenario, the regenerative process fails and is dominated by an unproductive inflammatory response and accompanying fibrosis. The failure of current regenerative therapeutics to completely restore functional muscle tissue is not surprising considering the incomplete understanding of the cellular mechanisms that drive the regeneration response in the setting of VML injury. To begin to address this profound knowledge gap, we developed an agent-based model to predict the tissue remodeling response following surgical creation of a VML injury. Once the model was able to recapitulate key aspects of the tissue remodeling response in the absence of repair, we validated the model by simulating the tissue remodeling response to VML injury following implantation of either a decellularized extracellular matrix scaffold or a minced muscle graft. The model suggested that the SSC microenvironment and absence of pro-differentiation SSC signals were the most important aspects of failed muscle regeneration in VML injuries. The major implication of this work is that agent-based models may provide a much-needed predictive tool to optimize the design of new therapies, and thereby, accelerate the clinical translation of regenerative therapeutics for VML injuries.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Passipieri JA, Christ GJ. The potential of combination therapeutics for more complete repair of volumetric muscle loss injuries: The role of exogenous growth factors and/or progenitor cells in implantable skeletal muscle tissue engineering technologies. Cells Tissues Organs 2016; 202:202–213. 10.1159/000447323 - DOI - PubMed
-
- Karalaki M, Fili S, Philippou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo 2009; 23:779–96. - PubMed
-
- Carlson BM, Faulkner JA. The regeneration of skeletal muscle fibers following injury: a review. Med. Sci. Sports Exerc. 1983; 15:187–198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

