Virus shedding kinetics and unconventional virulence tradeoffs
- PMID: 33970967
- PMCID: PMC8109835
- DOI: 10.1371/journal.ppat.1009528
Virus shedding kinetics and unconventional virulence tradeoffs
Abstract
Tradeoff theory, which postulates that virulence provides both transmission costs and benefits for pathogens, has become widely adopted by the scientific community. Although theoretical literature exploring virulence-tradeoffs is vast, empirical studies validating various assumptions still remain sparse. In particular, truncation of transmission duration as a cost of virulence has been difficult to quantify with robust controlled in vivo studies. We sought to fill this knowledge gap by investigating how transmission rate and duration were associated with virulence for infectious hematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss). Using host mortality to quantify virulence and viral shedding to quantify transmission, we found that IHNV did not conform to classical tradeoff theory. More virulent genotypes of the virus were found to have longer transmission durations due to lower recovery rates of infected hosts, but the relationship was not saturating as assumed by tradeoff theory. Furthermore, the impact of host mortality on limiting transmission duration was minimal and greatly outweighed by recovery. Transmission rate differences between high and low virulence genotypes were also small and inconsistent. Ultimately, more virulent genotypes were found to have the overall fitness advantage, and there was no apparent constraint on the evolution of increased virulence for IHNV. However, using a mathematical model parameterized with experimental data, it was found that host culling resurrected the virulence tradeoff and provided low virulence genotypes with the advantage. Human-induced or natural culling, as well as host population fragmentation, may be some of the mechanisms by which virulence diversity is maintained in nature. This work highlights the importance of considering non-classical virulence tradeoffs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

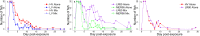
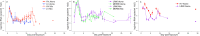

References
-
- Kurath G, Wargo AR. Evolution of viral virulence: empirical studies. In: Weaver SC, Denison M, Roossinck M, Vignuzzi M, editors. Virus evolution: current research and future directions. Portland, OR: Caister Academic Press; 2016. p. 155–214.
-
- Schmid-Hempel P. Virulence. In: Presss OU, editor. Evolutionary Parasitology. 312–353. New York: Oxford University Press; 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

