Distinct brain-derived TDP-43 strains from FTLD-TDP subtypes induce diverse morphological TDP-43 aggregates and spreading patterns in vitro and in vivo
- PMID: 33971027
- PMCID: PMC8578586
- DOI: 10.1111/nan.12732
Distinct brain-derived TDP-43 strains from FTLD-TDP subtypes induce diverse morphological TDP-43 aggregates and spreading patterns in vitro and in vivo
Abstract
Aim: The heterogeneity in the distribution and morphological features of TAR DNA-binding protein-43 (TDP-43) pathology in the brains of frontotemporal lobar degeneration (FTLD-TDP) patients and their different clinical manifestations suggest that distinct pathological TDP-43 strains could play a role in this heterogeneity between different FTLD-TDP subtypes (A-E). Our aim was to evaluate the existence of distinct TDP-43 strains in the brains of different FTLD-TDP subtypes and characterise their specific seeding properties in vitro and in vivo.
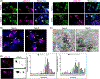
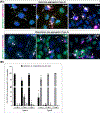
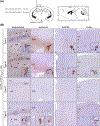
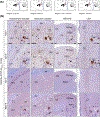
Methods and results: We used an inducible stable cell line expressing a mutant cytoplasmic TDP-43 (iGFP-NLSm) to evaluate the seeding properties of distinct pathological TDP-43 strains. Brain-derived TDP-43 protein extracts from FTLD-TDP types A (n = 6) and B (n = 3) cases induced the formation of round/spherical phosphorylated TDP-43 aggregates that morphologically differed from the linear and wavy wisps and bigger heterogeneous filamentous (skein-like) aggregates induced by type E (n = 3) cases. These morphological differences correlated with distinct biochemical banding patterns of sarkosyl-insoluble TDP-43 protein recovered from the transduced cells. Moreover, brain-derived TDP-43 extracts from type E cases showed higher susceptibility to PK digestion of full-length TDP-43 and the most abundant C-terminal fragments that characterise type E extracts. Finally, we showed that intracerebral injections of different TDP-43 strains induced a distinctive morphological and subcellular distribution of TDP-43 pathology and different spreading patterns in the brains of CamKIIa-hTDP-43NLSm Tg mice.
Conclusions: We show the existence of distinct TDP-43 strains in the brain of different FTLD-TDP subtypes with distinctive seeding and spreading properties in the brains of experimental animal models.
Keywords: TDP-43; frontotemporal lobar degeneration (FTLD); protein strains; seeding; spreading.
© 2021 British Neuropathological Society.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing interests.
Figures


References
-
- Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. - PubMed
-
- Forman MS, Mackenzie IR, Cairns NJ, et al. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65(6):571–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

