Efficacy of elobixibat as bowel preparation agent for colonoscopy: Prospective, randomized, multi-center study
- PMID: 33971037
- PMCID: PMC9290049
- DOI: 10.1111/den.14010
Efficacy of elobixibat as bowel preparation agent for colonoscopy: Prospective, randomized, multi-center study
Abstract
Background and aim: Elobixibat is a novel ileal bile acid transporter inhibitor. This study aimed to compare the efficacy, tolerability, and safety of the combination of elobixibat and 1 L of polyethylene glycol formulation containing ascorbic acid (PEG-Asc) solution versus the combination of sodium picosulfate and 1-L PEG-Asc solution as bowel preparation for colonoscopy.
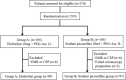
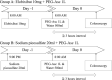
Methods: This multi-center, randomized, observer-blinded, non-inferiority study recruited 210 outpatients who were assigned to either the elobixibat plus 1-L PEG-Asc group (group A) or the sodium picosulfate plus 1-L PEG-Asc group (group B). The quality of the bowel cleansing level was assessed by the Boston Bowel Preparation Scale (BBPS) and compared the bowel cleansing level between the groups. Data regarding bowel preparation time, patients' tolerability, and adverse events were also analyzed.
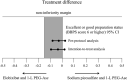
Results: Data for 196 patients (99 in group A and 97 in group B) were analyzed finally. BBPS was comparable between group A and B (8.3 ± 0.9 vs. 8.3 ± 0.7; P = 0.88). Consequently, the adequate bowel preparation rate in groups A and B was 95.0% and 99.0%, respectively (-4.0%, 95% CI -9.3 to 1.5). Bowel preparation time in group A was similar to that in group B (348.2 ± 79.8 min vs. 330.8 ± 82.5 min; P = 0.13), whereas, sleep disturbance was significantly less frequent in group A than in group B (10.2% vs. 22.7%; P = 0.02).
Conclusions: The combination of elobixibat and 1-L PEG-Asc can be considered an alternative bowel preparation for colonoscopy considering the equivalent bowel cleansing effect and less frequent sleep disturbance. The Japan Registry of Clinical Trials (jRCTs41180026).
Keywords: bowel cleansing; bowel preparation; colonoscopy; elobixibat; tolerability.
© 2021 The Authors. Digestive Endoscopy published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
Authors declare no conflict of interest for this article.
Figures

Similar articles
-
Comparison of the efficacy and tolerability of elobixibat plus sodium picosulfate with magnesium citrate and split-dose 2-L polyethylene glycol with ascorbic acid for bowel preparation before outpatient colonoscopy: a study protocol for the multicentre, randomised, controlled E-PLUS trial.BMC Gastroenterol. 2024 Feb 3;24(1):61. doi: 10.1186/s12876-024-03146-6. BMC Gastroenterol. 2024. PMID: 38310266 Free PMC article.
-
Comparison of a split-dose bowel preparation with 2 liters of polyethylene glycol plus ascorbic acid and 1 liter of polyethylene glycol plus ascorbic acid and bisacodyl before colonoscopy.Gastrointest Endosc. 2017 Aug;86(2):343-348. doi: 10.1016/j.gie.2016.10.040. Epub 2016 Nov 23. Gastrointest Endosc. 2017. PMID: 27889546 Clinical Trial.
-
Bowel Preparation Efficacy and Safety of 1 L vs 2 L Polyethylene Glycol With Ascorbic Acid for Colonoscopy: A Randomized Controlled Trial.Clin Transl Gastroenterol. 2023 Mar 1;14(3):e00532. doi: 10.14309/ctg.0000000000000532. Clin Transl Gastroenterol. 2023. PMID: 36113016 Free PMC article. Clinical Trial.
-
Cleaning effect and tolerance of 16 bowel preparation regimens on adult patients before colonoscopy: a network meta-analysis.Int J Colorectal Dis. 2023 Mar 11;38(1):69. doi: 10.1007/s00384-023-04355-3. Int J Colorectal Dis. 2023. PMID: 36905434 Review.
-
Comparative Efficacy of 2 L Polyethylene Glycol Alone or With Ascorbic Acid vs. 4 L Polyethylene Glycol for Colonoscopy: A Systematic Review and Network Meta-Analysis of 12 Randomized Controlled Trials.Front Med (Lausanne). 2019 Aug 21;6:182. doi: 10.3389/fmed.2019.00182. eCollection 2019. Front Med (Lausanne). 2019. PMID: 31497604 Free PMC article.
Cited by
-
Comparing the bowel cleansing efficacy between sodium picosulfate vs. 2L polyethylene glycol electrolyte lavage solution for colonoscopy: a prospective observational study.BMC Gastroenterol. 2025 Mar 12;25(1):164. doi: 10.1186/s12876-025-03707-3. BMC Gastroenterol. 2025. PMID: 40075296 Free PMC article.
-
Comparison of the efficacy and tolerability of elobixibat plus sodium picosulfate with magnesium citrate and split-dose 2-L polyethylene glycol with ascorbic acid for bowel preparation before outpatient colonoscopy: a study protocol for the multicentre, randomised, controlled E-PLUS trial.BMC Gastroenterol. 2024 Feb 3;24(1):61. doi: 10.1186/s12876-024-03146-6. BMC Gastroenterol. 2024. PMID: 38310266 Free PMC article.
References
-
- Kang SH, Jeen YT, Lee JH et al. Comparison of a split‐dose bowel preparation with 2 liters of polyethylene glycol plus ascorbic acid and 1 liter of polyethylene glycol plus ascorbic acid and bisacodyl before colonoscopy. Gastrointest Endosc 2017; 86: 343–8. - PubMed
-
- Gentile M, De Rosa M, Cestaro G, Forestieri P. 2 L PEG plus ascorbic acid versus 4 L PEG plus simethicon for colonoscopy preparation: A randomized single‐blind clinical trial. Surg Laparosc Endosc Percutan Tech 2013; 23: 276–80. - PubMed
-
- Valiante F, Pontone S, Hassan C et al. A randomized controlled trial evaluating a new 2‐L PEG solution plus ascorbic acid vs 4‐L PEG for bowel cleansing prior to colonoscopy. Dig Liver Dis 2012; 44: 224–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

