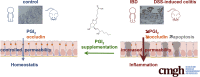
PGI2 Inhibits Intestinal Epithelial Permeability and Apoptosis to Alleviate Colitis
- PMID: 33971327
- PMCID: PMC8342971
- DOI: 10.1016/j.jcmgh.2021.05.001
PGI2 Inhibits Intestinal Epithelial Permeability and Apoptosis to Alleviate Colitis
Abstract
Background & aims: Inflammatory bowel diseases (IBDs) that encompass both ulcerative colitis and Crohn's disease are a major public health problem with an etiology that has not been fully elucidated. There is a need to improve disease outcomes and preventive measures by developing new effective and lasting treatments. Although polyunsaturated fatty acid metabolites play an important role in the pathogenesis of several disorders, their contribution to IBD is yet to be understood.
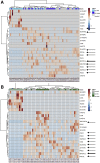
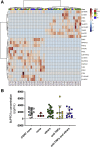
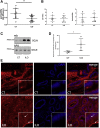
Methods: Polyunsaturated fatty acids metabolite profiles were established from biopsy samples obtained from Crohn's disease, ulcerative colitis, or control patients. The impact of a prostaglandin I2 (PGI2) analog on intestinal epithelial permeability was tested in vitro using Caco-2 cells and ex vivo using human or mouse explants. In addition, mice were treated with PGI2 to observe dextran sulfate sodium (DSS)-induced colitis. Tight junction protein expression, subcellular location, and apoptosis were measured in the different models by immunohistochemistry and Western blotting.
Results: A significant reduction of PGI2 in IBD patient biopsies was identified. PGI2 treatment reduced colonic inflammation, increased occludin expression, decreased caspase-3 cleavage and intestinal permeability, and prevented colitis development in DSS-induced mice. Using colonic explants from mouse and human control subjects, the staurosporine-induced increase in paracellular permeability was prevented by PGI2. PGI2 also induced the membrane location of occludin and reduced the permeability observed in colonic biopsies from IBD patients.
Conclusions: The present study identified a PGI2 defect in the intestinal mucosa of IBD patients and demonstrated its protective role during colitis.
Keywords: Caspase-3; Human Mucosa; IBD; Lipidomic; Occludin; Omega-6 (n-6); PGI(2).
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures








References
-
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., Kaplan G.G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54 e42. quiz e30. - PubMed
-
- Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nature Reviews Gastroenterology Hepatology. 2015;12:205–217. - PubMed
-
- Gu Y.B., Zhong J. Endoscopic management of stricturing Crohn’s disease. J Dig Dis. 2020;21:351–354. - PubMed
-
- Spinelli A., Armuzzi A., Ciccocioppo R., Danese S., Gionchetti P., Luglio G., Orlando A., Rispo A., Rizzello F., Sofo L., Solina G., Poggioli G. Management of patients with complex perianal fistulas in Crohn’s disease: optimal patient flow in the Italian clinical reality. Dig Liver Dis. 2020;52:506–515. - PubMed
-
- Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

