Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection
- PMID: 33971404
- PMCID: PMC8104913
- DOI: 10.1016/j.ebiom.2021.103369
Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection
Abstract
Background: Coronavirus-2 (SARS-CoV-2) infection causes an acute respiratory syndrome accompanied by multi-organ damage that implicates a prothrombotic state leading to widespread microvascular clots. The causes of such coagulation abnormalities are unknown. The receptor tissue factor, also known as CD142, is often associated with cell-released extracellular vesicles (EV). In this study, we aimed to characterize surface antigens profile of circulating EV in COVID-19 patients and their potential implication as procoagulant agents.
Methods: We analyzed serum-derived EV from 67 participants who underwent nasopharyngeal swabs molecular test for suspected SARS-CoV-2 infection (34 positives and 33 negatives) and from 16 healthy controls (HC), as referral. A sub-analysis was performed on subjects who developed pneumonia (n = 28). Serum-derived EV were characterized for their surface antigen profile and tested for their procoagulant activity. A validation experiment was performed pre-treating EV with anti-CD142 antibody or with recombinant FVIIa. Serum TNF-α levels were measured by ELISA.
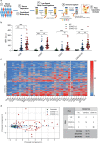
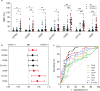
Findings: Profiling of EV antigens revealed a surface marker signature that defines circulating EV in COVID-19. A combination of seven surface molecules (CD49e, CD209, CD86, CD133/1, CD69, CD142, and CD20) clustered COVID (+) versus COVID (-) patients and HC. CD142 showed the highest discriminating performance at both multivariate models and ROC curve analysis. Noteworthy, we found that CD142 exposed onto surface of EV was biologically active. CD142 activity was higher in COVID (+) patients and correlated with TNF-α serum levels.
Interpretation: In SARS-CoV-2 infection the systemic inflammatory response results in cell-release of substantial amounts of procoagulant EV that may act as clotting initiation agents, contributing to disease severity.
Funding: Cardiocentro Ticino Institute, Ente ospedaliero Cantonale, Lugano-Switzerland.
Keywords: Coagulation; Extracellular vesicles; Pneumonia; SARS-CoV-2; Tissue factor.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Authors have nothing to disclose.
Figures


Comment in
-
Procoagulant activity of extracellular vesicles in plasma of patients with SARS-CoV-2 infection.EBioMedicine. 2021 Jun;68:103411. doi: 10.1016/j.ebiom.2021.103411. Epub 2021 Jun 3. EBioMedicine. 2021. PMID: 34091416 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

