Exploiting the HSP60/10 chaperonin system as a chemotherapeutic target for colorectal cancer
- PMID: 33971488
- PMCID: PMC8194340
- DOI: 10.1016/j.bmc.2021.116129
Exploiting the HSP60/10 chaperonin system as a chemotherapeutic target for colorectal cancer
Abstract
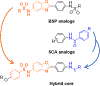
Over the past few decades, an increasing variety of molecular chaperones have been investigated for their role in tumorigenesis and as potential chemotherapeutic targets; however, the 60 kDa Heat Shock Protein (HSP60), along with its HSP10 co-chaperone, have received little attention in this regard. In the present study, we investigated two series of our previously developed inhibitors of the bacterial homolog of HSP60/10, called GroEL/ES, for their selective cytotoxicity to cancerous over non-cancerous colorectal cells. We further developed a third "hybrid" series of analogs to identify new candidates with superior properties than the two parent scaffolds. Using a series of well-established HSP60/10 biochemical screens and cell-viability assays, we identified 24 inhibitors (14%) that exhibited > 3-fold selectivity for targeting colorectal cancer over non-cancerous cells. Notably, cell viability EC50 results correlated with the relative expression of HSP60 in the mitochondria, suggesting a potential for this HSP60-targeting chemotherapeutic strategy as emerging evidence indicates that HSP60 is up-regulated in colorectal cancer tumors. Further examination of five lead candidates indicated their ability to inhibit the clonogenicity and migration of colorectal cancer cells. These promising results are the most thorough analysis and first reported instance of HSP60/10 inhibitors being able to selectively target colorectal cancer cells and highlight the potential of the HSP60/10 chaperonin system as a viable chemotherapeutic target.
Keywords: Chaperonin; Chemotherapeutic; Colorectal cancer; GroEL; GroES; HSP10; HSP60; Molecular chaperone; Proteostasis; Small molecule inhibitors.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare the following competing financial interest(s): S.M.J. and E.C. are co-founders of BioEL Inc, which was formed to commercialize GroEL/ES inhibitors, stemming from research conducted in their laboratories, for antibacterial applications.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures






References
-
- Ferlay J; Ervik M; Lam F; Colombet M; Mery L; Pineros M; Znaor A; Soerjomataram I; Bray F Cancer Today (powered by GLOBOCAN 2018) http://publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Powe... (accessed Jul 11, 2019).
-
- Howlander N; Noone AM; Krapcho M; Miller D; Brest A; Yu M; Ruhl J; Tatalovich Z; Mariotto A; Lewis DR; et al. SEER Cancer Statistics Review 1975–2016. Natl. Cancer Institute. 2019, https://seer.cancer.gov/csr/1975_2016/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Research Materials
Miscellaneous

