Novel leukocyte-depleted platelet-rich plasma-based skin equivalent as an in vitro model of chronic wounds: a preliminary study
- PMID: 33971814
- PMCID: PMC8111747
- DOI: 10.1186/s12860-021-00366-6
Novel leukocyte-depleted platelet-rich plasma-based skin equivalent as an in vitro model of chronic wounds: a preliminary study
Abstract
Background: Chronic leg ulcerations are associated with Haemoglobin disorders, Type2 Diabetes Mellitus, and long-term venous insufficiency, where poor perfusion and altered metabolism develop into a chronic inflammation that impairs wound closure. Skin equivalent organotypic cultures can be engineered in vitro to study skin biology and wound closure by modelling the specific cellular components of the skin. This study aimed to develop a novel bioactive platelet-rich plasma (PRP) leukocyte depleted scaffold to facilitate the study of common clinical skin wounds in patients with poor chronic skin perfusion and low leukocyte infiltration. A scratch assay was performed on the skin model to mimic two skin wound conditions, an untreated condition and a condition treated with recombinant tumour necrotic factor (rTNF) to imitate the stimulation of an inflammatory state. Gene expression of IL8 and TGFA was analysed in both conditions. Statistical analysis was done through ANOVA and paired student t-test. P < 0.05 was considered significant.
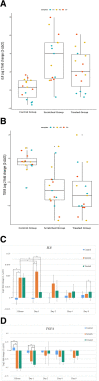
Results: A skin model that consisted of a leukocyte-depleted, platelet-rich plasma scaffold was setup with embedded fibroblasts as dermal equivalents and seeded keratinocytes as multi-layered epidermis. Gene expression levels of IL8 and TGFA were significantly different between the control and scratched conditions (p < 0.001 and p < 0.01 respectively), as well as between the control and treated conditions (p < 0.01 and p < 0.001 respectively). The scratch assay induced IL8 upregulation after 3 h (p < 0.05) which continued to increase up to day 1 (p < 0.05). On the other hand, the administration of TNF led to the downregulation of IL8 (p < 0.01), followed by an upregulation on day 2. IL8 gene expression decreased in the scratched condition after day 1 as the natural healing process took place and was lower than in the treated condition on day 8 (p < 0.05). Both untreated and treated conditions showed a downregulation of TGFA 3 h after scratch when compared with the control condition (p < 0.01). Administration of rTNF showed significant downregulation of TGFA after 24 h when compared with the control (p < 0.01) and treated conditions (p < 0.05).
Conclusion: This study suggests that a leukocyte-depleted PRP-based skin equivalent can be a useful model for the in vitro study of chronic skin wounds related to poor skin perfusion.
Keywords: Biomaterials; Inflammation; Platelet rich plasma; Skin equivalent; Wound healing.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Platelet-rich plasma with keratinocytes and fibroblasts enhance healing of full-thickness wounds.J Tissue Viability. 2017 Aug;26(3):208-215. doi: 10.1016/j.jtv.2017.05.003. Epub 2017 Jun 7. J Tissue Viability. 2017. PMID: 28615133
-
Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing.Cytotherapy. 2015 Mar;17(3):293-300. doi: 10.1016/j.jcyt.2014.10.005. Epub 2014 Nov 21. Cytotherapy. 2015. PMID: 25456581
-
Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro.Arch Dermatol Res. 2016 Sep;308(7):511-20. doi: 10.1007/s00403-016-1676-1. Epub 2016 Jul 9. Arch Dermatol Res. 2016. PMID: 27394438
-
L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds.Curr Pharm Biotechnol. 2012 Jun;13(7):1266-77. doi: 10.2174/138920112800624463. Curr Pharm Biotechnol. 2012. PMID: 21740368 Review.
-
Upregulation of tumor suppressor protein neurofibromin in normal human wound healing and in vitro evidence for platelet derived growth factor (PDGF) and transforming growth factor-beta1 (TGF-beta1) elicited increase in neurofibromin mRNA steady-state levels in dermal fibroblasts.J Invest Dermatol. 1998 Mar;110(3):232-7. doi: 10.1046/j.1523-1747.1998.00108.x. J Invest Dermatol. 1998. PMID: 9506441 Review.
Cited by
-
A comparative profile of total protein and six angiogenically-active growth factors in three platelet products.GMS Interdiscip Plast Reconstr Surg DGPW. 2022 Jul 5;11:Doc06. doi: 10.3205/iprs000167. eCollection 2022. GMS Interdiscip Plast Reconstr Surg DGPW. 2022. PMID: 35909816 Free PMC article.
-
Tissue Engineering as a Promising Treatment for Glottic Insufficiency: A Review on Biomolecules and Cell-Laden Hydrogel.Biomedicines. 2022 Nov 30;10(12):3082. doi: 10.3390/biomedicines10123082. Biomedicines. 2022. PMID: 36551838 Free PMC article. Review.
-
Possible Role of Circulating Bone Marrow Mesenchymal Progenitors in Modulating Inflammation and Promoting Wound Repair.Int J Mol Sci. 2021 Dec 22;23(1):78. doi: 10.3390/ijms23010078. Int J Mol Sci. 2021. PMID: 35008501 Free PMC article.
-
Autologous Fat Plus Platelet-Rich Plasma versus Autologous Fat Alone on Sulcus Vocalis.J Clin Med. 2022 Jan 29;11(3):725. doi: 10.3390/jcm11030725. J Clin Med. 2022. PMID: 35160180 Free PMC article.
References
-
- Scerri CA, Abela W, Galdies R, Pizzuto M, Grech JL, Felice AE. The beta + IVS, I-NT no. 6 (T --> C) thalassaemia in heterozygotes with an associated Hb Valletta or Hb S heterozygosity in homozygotes from Malta. Br J Haematol. 1993;83(4):669–671. doi: 10.1111/j.1365-2141.1993.tb04710.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

