Impact of relative dose intensity of oxaliplatin in adjuvant therapy among stage III colon cancer patients on early recurrence: a retrospective cohort study
- PMID: 33971834
- PMCID: PMC8112028
- DOI: 10.1186/s12885-021-08183-y
Impact of relative dose intensity of oxaliplatin in adjuvant therapy among stage III colon cancer patients on early recurrence: a retrospective cohort study
Abstract
Background: Oxaliplatin-based therapy with FOLFOX-4 or CAPOX administered over 6 months remains the standard adjuvant treatment for stage III colon cancer (CC) patients. However, many patients experience dose reduction or early termination of chemotherapy due to oxaliplatin toxicity, which may increase the risk of early recurrence. The objective of this study was to analyze the relationship between the relative dose intensity of oxaliplatin (RDI-O) and early recurrence among stage III CC patients.
Methods: The study included 365 patients treated at five oncology centers in Poland between 2000 and 2014. Survival analysis was performed using the Kaplan-Meier method. Univariate analysis was performed using the Cox proportional hazard model; multivariate analysis was performed with the stepwise forward approach. For all analyses the α level of 0.05 was employed.
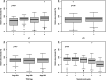
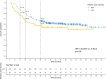
Results: The median follow-up was 51.8 months (range 8.2-115.1). Early recurrence < 36 months after surgery occurred in 130 patients (37.8%). In this group 51 (39.2%) and 87 (66.9%) of patients were low and high-risk, respectively. Receipt < 60% of RDI-O was associated with early recurrence within 18 months after surgery (OR = 2.05; 95%CI: 1.18-3.51; p = 0.010), especially in low-risk group (HR = 1.56 (95%CI: 0.96-2.53), p = 0.07). In the multivariate analysis early recurrence was correlated with grade (OR = 2.47; 95% CI: 1.25-4.8; p = 0.008), pN (OR = 2.63; 95% CI: 1.55-4.54; p < 0.001), the number of lymph nodes harvested (OR = 0.51; 95% CI: 0.29-0.86; p = 0.013) and RDI-O (OR = 1.91; 95%CI: 1.06-3.39; p = 0.028). The early vs. late recurrence negatively correlated with OS regardless of the RDI-O (HR = 22.9 (95%CI: 13.9-37.6; p < 0.001).
Conclusions: RDI-O < 60% in adjuvant therapy among stage III CC (especially in low-risk group) increases the risk of early recurrence within 18 months of surgery. Patients with early recurrence showed worse overall survival regardless of the RDI-O.
Keywords: Adjuvant chemotherapy; Colon cancer; Cumulative dose; Oxaliplatin; Relative dose intensity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. - DOI - PubMed
-
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. - DOI - PubMed
-
- Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. - DOI - PubMed
-
- Schmoll HJ, Tabernero J, Maround J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33(32):3733–3740. doi: 10.1200/JCO.2015.60.9107. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

