Benefit of switching to mepolizumab from omalizumab in severe eosinophilic asthma based on patient characteristics
- PMID: 33971856
- PMCID: PMC8111733
- DOI: 10.1186/s12931-021-01733-9
Benefit of switching to mepolizumab from omalizumab in severe eosinophilic asthma based on patient characteristics
Abstract
Background: The OSMO study assessed the efficacy of switching to mepolizumab in patients with severe eosinophilic asthma that was uncontrolled whilst receiving omalizumab. The objective of this analysis was to assess the proportion of patients achieving pre-defined improvements in up to four efficacy outcomes and the relationship between patient baseline characteristics and treatment response.
Methods: This was a post hoc analysis of OSMO study data (GSK ID:204471; ClinicalTrials.gov No. NCT02654145). Patients with severe eosinophilic asthma uncontrolled by high-dose inhaled corticosteroids, other controller(s) and omalizumab subcutaneously (≥ 4 months) were switched to mepolizumab 100 mg administered subcutaneously. Endpoints included the proportion of responders-i.e. patients achieving a pre-defined clinical improvement in ≥ 1 of the following outcomes: (1) Asthma Control Questionnaire (ACQ)-5 score (≥ 0.5-points), (2) St George's Respiratory Questionnaire (SGRQ) total score (≥ 4-points), (3) pre-bronchodilator forced expiratory volume in 1s (FEV1; ≥ 100 mL), all at Week 32, and (4) annualised rate of clinically significant exacerbations (≥ 50% reduction).
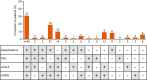
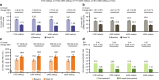
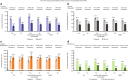
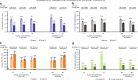
Results: Of the 145 patients included, 94%, 83%, 63% and 31% were responders for ≥ 1, ≥ 2, ≥ 3 and 4 outcomes, respectively; 75% and 78% were ACQ-5 and SGRQ score responders, and 50% and 69% were FEV1 and exacerbation responders. Subgroup analyses demonstrated improvements irrespective of baseline blood eosinophil count, prior omalizumab treatment regimen/duration, comorbidities, prior exacerbation history, maintenance oral corticosteroid use, ACQ-5 and SGRQ scores, and body weight/body mass index.
Conclusions: After switching to mepolizumab, almost all patients with uncontrolled severe eosinophilic asthma on omalizumab achieved a beneficial response in ≥ 1 clinical outcome. Improvements were observed regardless of baseline characteristics. Trial registration This manuscript is a post hoc analysis of data from the OSMO study. ClinicalTrials.gov, NCT02654145. Registered January 13, 2016.
Keywords: Asthma; Asthma treatment; Biologics; Eosinophils.
Conflict of interest statement
SGS, JA, DM, and RGP are all employees of GSK and hold stocks/shares in GSK. FCA is a former employee of GSK and holds GSK stocks/shares and is currently employed by Avillion US, Inc. DVG is a former employee of GSK and holds GSK stock/shares and is currently employed by Chiesi USA; KRC has received consulting fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, GSK, Grifols, Kamada, Novartis, Roche, and Sanofi Regeneron; has undertaken research funded by Amgen, AstraZeneca, Boehringer Ingelheim, CSL Behring, GSK, Grifols, Kamada, Novartis, Roche and Sanofi; has participated in continuing medical education activities sponsored in whole or in part by AstraZeneca, Boehringer Ingelheim, GSK, Grifols, Novartis and Teva. KRC is also participating in research funded by the Canadian Institutes of Health Research. GD has received personal fees or research grants from Novartis Pharma, AstraZeneca, GSK, Boehringer Ingelheim, Mundi Pharma, Vivisol, Sanofi, Chiesi, ALK, Teva, MSD and AGIR à Dom; has participated in CME activities sponsored by GSK, AstraZeneca, Novartis Pharma, Chiesi, MSD, Takeda, AGIR à Dom, Orkyn, Mundi Pharma, ALK, Stallergenes, Boehringer Ingelheim and Teva; is participating in research funded by GSK, ALK, AstraZeneca, Novartis Pharma, Boehringer Ingelheim, Vitalair, AB science, Amgen, Lilly, Sanofi, Roche and Teva. MB has received personal fees or research grants from AstraZeneca, Novartis, GSK, Boehringer Ingelheim, and Sanofi. MCL has participated in advisory board meetings for GSK, Gossamer Bio and AstraZeneca, and has received research grants from GSK, and Gossamer Bio. BC is an advisor for, has received consultancy fees from, and is on the speaker's bureau AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Regeneron and Sanofi-Genzyme. XM has received fees as a speaker, scientific advisor or participant of clinical studies for AstraZeneca, Boehringer Ingelheim, Chiesi, Faes, GSK, Menarini, MundiPharma, Novartis and Teva.
Figures

References
-
- GSK UK. Mepolizumab (NUCALA) EU summary of product characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/nucala-epar-p.... Accessed 3 Nov 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

