Transposition of cardiovascular outcome trial effects to the real-world population of patients with type 2 diabetes
- PMID: 33971880
- PMCID: PMC8112047
- DOI: 10.1186/s12933-021-01300-y
Transposition of cardiovascular outcome trial effects to the real-world population of patients with type 2 diabetes
Abstract
Background: Transferring results obtained in cardiovascular outcome trials (CVOTs) to the real-world setting is challenging. We herein transposed CVOT results to the population of patients with type 2 diabetes (T2D) seen in routine clinical practice and who may receive the medications tested in CVOTs.
Methods: We implemented the post-stratification approach based on aggregate data of CVOTs and individual data of a target population of diabetic outpatients. We used stratum-specific estimates available from CVOTs to calculate expected effect size for the target population by weighting the average of the stratum-specific treatment effects according to proportions of a given characteristic in the target population. Data are presented as hazard ratio (HR) and 95% confidence intervals.
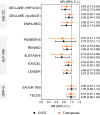
Results: Compared to the target population (n = 139,708), the CVOT population (n = 95,816) was younger and had a two to threefold greater prevalence of cardiovascular disease. EMPA-REG was the CVOT with the largest variety of details on stratum-specific effects, followed by TECOS, whereas DECLARE and PIONEER-6 had more limited stratum-specific information. The post-stratification HR estimate for 3 point major adverse cardiovascular event (MACE) based on EMPA-REG was 0.88 (0.74-1.03) in the target population, compared to 0.86 (0.74-0.99) in the trial. The HR estimate based on LEADER was 0.88 (0.77-0.99) in the target population compared to 0.87 (0.78-0.97) in the trial. Consistent results were obtained for SUSTAIN-6, EXSCEL, PIONEER-6 and DECLARE. The effect of DPP-4 inhibitors observed in CVOTs remained neutral in the target population.
Conclusions: Based on CVOT stratum-specific effects, cardiovascular protective actions of glucose lowering medications tested in CVOTs are transferrable to a much different real-world population of patients with T2D.
Conflict of interest statement
AA received research grants, lecture or advisory board fees from: Merck Sharp & Dome, AstraZeneca, Novartis, Boehringer-Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, and Takeda. GPF received grants, honoraria or lecture fees from Abbott, Astrazeneca, Boehringer, Lilly, Novo Nordisk, Sanofi. VS and PB have nothing to disclose.
Figures

References
-
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. - DOI - PMC - PubMed
-
- Sharma A, Pagidipati NJ, Califf RM, McGuire DK, Green JB, Demets D, George JT, Gerstein HC, Hobbs T, Holman RR, et al. Impact of regulatory guidance on evaluating cardiovascular risk of new glucose-lowering therapies to treat type 2 diabetes mellitus: lessons learned and future directions. Circulation. 2020;141(10):843–862. doi: 10.1161/CIRCULATIONAHA.119.041022. - DOI - PubMed
-
- Ghosh-Swaby OR, Goodman SG, Leiter LA, Cheng A, Connelly KA, Fitchett D, Juni P, Farkouh ME, Udell JA. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2020;8(5):418–435. doi: 10.1016/S2213-8587(20)30038-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

