Impact of Gba2 on neuronopathic Gaucher's disease and α-synuclein accumulation in medaka (Oryzias latipes)
- PMID: 33971917
- PMCID: PMC8111776
- DOI: 10.1186/s13041-021-00790-x
Impact of Gba2 on neuronopathic Gaucher's disease and α-synuclein accumulation in medaka (Oryzias latipes)
Abstract
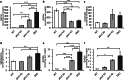
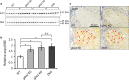
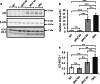
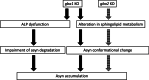
Homozygous mutations in the lysosomal glucocerebrosidase gene, GBA1, cause Gaucher's disease (GD), while heterozygous mutations in GBA1 are a strong risk factor for Parkinson's disease (PD), whose pathological hallmark is intraneuronal α-synuclein (asyn) aggregates. We previously reported that gba1 knockout (KO) medaka exhibited glucosylceramide accumulation and neuronopathic GD phenotypes, including short lifespan, the dopaminergic and noradrenergic neuronal cell loss, microglial activation, and swimming abnormality, with asyn accumulation in the brains. A recent study reported that deletion of GBA2, non-lysosomal glucocerebrosidase, in a non-neuronopathic GD mouse model rescued its phenotypes. In the present study, we generated gba2 KO medaka and examined the effect of Gba2 deletion on the phenotypes of gba1 KO medaka. The Gba2 deletion in gba1 KO medaka resulted in the exacerbation of glucosylceramide accumulation and no improvement in neuronopathic GD pathological changes, asyn accumulation, or swimming abnormalities. Meanwhile, though gba2 KO medaka did not show any apparent phenotypes, biochemical analysis revealed asyn accumulation in the brains. gba2 KO medaka showed a trend towards an increase in sphingolipids in the brains, which is one of the possible causes of asyn accumulation. In conclusion, this study demonstrated that the deletion of Gba2 does not rescue the pathological changes or behavioral abnormalities of gba1 KO medaka, and GBA2 represents a novel factor affecting asyn accumulation in the brains.
Keywords: GBA1; GBA2; Gaucher’s disease; Glucocerebrosidase; Parkinson’s disease; Sphingolipids; α-Synuclein.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Viable neuronopathic Gaucher disease model in Medaka (Oryzias latipes) displays axonal accumulation of alpha-synuclein.PLoS Genet. 2015 Apr 2;11(4):e1005065. doi: 10.1371/journal.pgen.1005065. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25835295 Free PMC article.
-
AAV delivery of GBA1 suppresses α-synuclein accumulation in Parkinson's disease models and restores functions in Gaucher's disease models.PLoS One. 2025 May 7;20(5):e0321145. doi: 10.1371/journal.pone.0321145. eCollection 2025. PLoS One. 2025. PMID: 40333681 Free PMC article.
-
Glucocerebrosidase 1 deficient Danio rerio mirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death.Hum Mol Genet. 2015 Dec 1;24(23):6640-52. doi: 10.1093/hmg/ddv369. Epub 2015 Sep 16. Hum Mol Genet. 2015. PMID: 26376862 Free PMC article.
-
Lysosomal trafficking defects link Parkinson's disease with Gaucher's disease.Mov Disord. 2016 Nov;31(11):1610-1618. doi: 10.1002/mds.26802. Epub 2016 Sep 13. Mov Disord. 2016. PMID: 27619775 Free PMC article. Review.
-
Molecular mechanisms of α-synuclein and GBA1 in Parkinson's disease.Cell Tissue Res. 2018 Jul;373(1):51-60. doi: 10.1007/s00441-017-2704-y. Epub 2017 Oct 24. Cell Tissue Res. 2018. PMID: 29064079 Free PMC article. Review.
Cited by
-
Teleost Fish and Organoids: Alternative Windows Into the Development of Healthy and Diseased Brains.Front Mol Neurosci. 2022 Aug 11;15:855786. doi: 10.3389/fnmol.2022.855786. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36034498 Free PMC article. Review.
-
A lipid nanoparticle-based oligodendrocyte-specific mRNA therapy.Mol Ther Nucleic Acids. 2024 Nov 5;35(4):102380. doi: 10.1016/j.omtn.2024.102380. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39640012 Free PMC article.
-
Application of CRISPR/Cas9 technology in the modeling of Gaucher disorder.Biochem Biophys Rep. 2024 Nov 19;40:101872. doi: 10.1016/j.bbrep.2024.101872. eCollection 2024 Dec. Biochem Biophys Rep. 2024. PMID: 39850539 Free PMC article. Review.
-
Animal Models for the Study of Gaucher Disease.Int J Mol Sci. 2023 Nov 7;24(22):16035. doi: 10.3390/ijms242216035. Int J Mol Sci. 2023. PMID: 38003227 Free PMC article. Review.
-
Distinct sets of lysosomal genes define synucleinopathy and tauopathy.BMB Rep. 2023 Dec;56(12):657-662. doi: 10.5483/BMBRep.2023-0109. BMB Rep. 2023. PMID: 37817435 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

