Endogenous retroviruses in the origins and treatment of cancer
- PMID: 33971937
- PMCID: PMC8108463
- DOI: 10.1186/s13059-021-02357-4
Endogenous retroviruses in the origins and treatment of cancer
Abstract
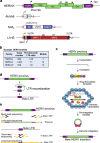
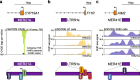
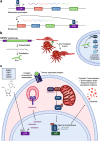
Endogenous retroviruses (ERVs) are emerging as promising therapeutic targets in cancer. As remnants of ancient retroviral infections, ERV-derived regulatory elements coordinate expression from gene networks, including those underpinning embryogenesis and immune cell function. ERV activation can promote an interferon response, a phenomenon termed viral mimicry. Although ERV expression is associated with cancer, and provisionally with autoimmune and neurodegenerative diseases, ERV-mediated inflammation is being explored as a way to sensitize tumors to immunotherapy. Here we review ERV co-option in development and innate immunity, the aberrant contribution of ERVs to tumorigenesis, and the wider biomedical potential of therapies directed at ERVs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Mager DL, Stoye JP. Mammalian endogenous retroviruses. Microbiol Spectr. 2015;3:1–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

