Early embryogenesis and organogenesis in the annelid Owenia fusiformis
- PMID: 33971947
- PMCID: PMC8111721
- DOI: 10.1186/s13227-021-00176-z
Early embryogenesis and organogenesis in the annelid Owenia fusiformis
Abstract
Background: Annelids are a diverse group of segmented worms within Spiralia, whose embryos exhibit spiral cleavage and a variety of larval forms. While most modern embryological studies focus on species with unequal spiral cleavage nested in Pleistoannelida (Sedentaria + Errantia), a few recent studies looked into Owenia fusiformis, a member of the sister group to all remaining annelids and thus a key lineage to understand annelid and spiralian evolution and development. However, the timing of early cleavage and detailed morphogenetic events leading to the formation of the idiosyncratic mitraria larva of O. fusiformis remain largely unexplored.
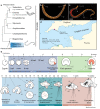
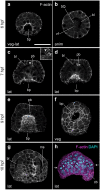
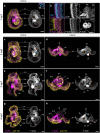
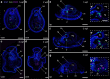
Results: Owenia fusiformis undergoes equal spiral cleavage where the first quartet of animal micromeres are slightly larger than the vegetal macromeres. Cleavage results in a coeloblastula approximately 5 h post-fertilization (hpf) at 19 °C. Gastrulation occurs via invagination and completes 4 h later, with putative mesodermal precursors and the chaetoblasts appearing 10 hpf at the dorso-posterior side. Soon after, at 11 hpf, the apical tuft emerges, followed by the first neurons (as revealed by the expression of elav1 and synaptotagmin-1) in the apical organ and the prototroch by 13 hpf. Muscles connecting the chaetal sac to various larval tissues develop around 18 hpf and by the time the mitraria is fully formed at 22 hpf, there are FMRFamide+ neurons in the apical organ and prototroch, the latter forming a prototrochal ring. As the mitraria feeds, it grows in size and the prototroch expands through active proliferation. The larva becomes competent after ~ 3 weeks post-fertilization at 15 °C, when a conspicuous juvenile rudiment has formed ventrally.
Conclusions: Owenia fusiformis embryogenesis is similar to that of other equal spiral cleaving annelids, supporting that equal cleavage is associated with the formation of a coeloblastula, gastrulation via invagination, and a feeding trochophore-like larva in Annelida. The nervous system of the mitraria larva forms earlier and is more elaborated than previously recognized and develops from anterior to posterior, which is likely an ancestral condition to Annelida. Altogether, our study identifies the major developmental events during O. fusiformis ontogeny, defining a conceptual framework for future investigations.
Keywords: Annelida; Equal cleavage; Larva; Mitraria; Nervous system; Neural development; Owenia fusiformis; Spiral cleavage; Spiralia; Trochophore.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The development of the adult nervous system in the annelid Owenia fusiformis.Neural Dev. 2024 Feb 21;19(1):3. doi: 10.1186/s13064-024-00180-8. Neural Dev. 2024. PMID: 38383501 Free PMC article.
-
Owenia fusiformis - a basally branching annelid suitable for studying ancestral features of annelid neural development.BMC Evol Biol. 2016 Jun 16;16(1):129. doi: 10.1186/s12862-016-0690-4. BMC Evol Biol. 2016. PMID: 27306767 Free PMC article.
-
Unusual development of the mitraria larva in the polychaete Owenia collaris.Biol Bull. 2009 Dec;217(3):253-68. doi: 10.1086/BBLv217n3p253. Biol Bull. 2009. PMID: 20040750
-
Sipuncula: an emerging model of spiralian development and evolution.Int J Dev Biol. 2014;58(6-8):485-99. doi: 10.1387/ijdb.140095mb. Int J Dev Biol. 2014. PMID: 25690964 Review.
-
Ins and outs of Spiralian gastrulation.Int J Dev Biol. 2014;58(6-8):413-28. doi: 10.1387/ijdb.140151dl. Int J Dev Biol. 2014. PMID: 25690959 Review.
Cited by
-
Duplication and Losses of Opsin Genes in Lophotrochozoan Evolution.Mol Biol Evol. 2023 Apr 4;40(4):msad066. doi: 10.1093/molbev/msad066. Mol Biol Evol. 2023. PMID: 36947081 Free PMC article.
-
Cell fate specification modes shape transcriptome evolution in the highly conserved spiral cleavage.EMBO Rep. 2025 Sep 4. doi: 10.1038/s44319-025-00569-4. Online ahead of print. EMBO Rep. 2025. PMID: 40908303
-
The development of the adult nervous system in the annelid Owenia fusiformis.Neural Dev. 2024 Feb 21;19(1):3. doi: 10.1186/s13064-024-00180-8. Neural Dev. 2024. PMID: 38383501 Free PMC article.
-
Morphological Observation and Transcriptome Analysis of Ciliogenesis in Urechis unicinctus (Annelida, Echiura).Int J Mol Sci. 2023 Jul 16;24(14):11537. doi: 10.3390/ijms241411537. Int J Mol Sci. 2023. PMID: 37511295 Free PMC article.
-
Postembryonic development and male paedomorphosis in Osedax (Siboglinidae, Annelida).Front Neurosci. 2024 Mar 18;18:1369274. doi: 10.3389/fnins.2024.1369274. eCollection 2024. Front Neurosci. 2024. PMID: 38562300 Free PMC article.
References
-
- Marlétaz F, Peijnenburg KTCA, Goto T, Satoh N, Rokhsar DS. A new spiralian Phylogeny places the enigmatic arrow worms among gnathiferans. Curr Biol. 2019;29(312–318):e3. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

