Novel chromosomal insertions of ISEcp1-blaCTX-M-15 and diverse antimicrobial resistance genes in Zambian clinical isolates of Enterobacter cloacae and Escherichia coli
- PMID: 33971966
- PMCID: PMC8111917
- DOI: 10.1186/s13756-021-00941-8
Novel chromosomal insertions of ISEcp1-blaCTX-M-15 and diverse antimicrobial resistance genes in Zambian clinical isolates of Enterobacter cloacae and Escherichia coli
Abstract
Background: The epidemiology of extended-spectrum β-lactamases (ESBLs) has undergone dramatic changes, with CTX-M-type enzymes prevailing over other types. blaCTX-M genes, encoding CTX-M-type ESBLs, are usually found on plasmids, but chromosomal location is becoming common. Given that blaCTX-M-harboring strains often exhibit multidrug resistance (MDR), it is important to investigate the association between chromosomally integrated blaCTX-M and the presence of additional antimicrobial resistance (AMR) genes, and to identify other relevant genetic elements.
Methods: A total of 46 clinical isolates of cefotaxime-resistant Enterobacteriaceae (1 Enterobacter cloacae, 9 Klebsiella pneumoniae, and 36 Escherichia coli) from Zambia were subjected to whole-genome sequencing (WGS) using MiSeq and MinION. By reconstructing nearly complete genomes, blaCTX-M genes were categorized as either chromosomal or plasmid-borne.
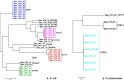
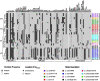
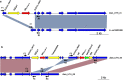
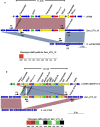
Results: WGS-based genotyping identified 58 AMR genes, including four blaCTX-M alleles (i.e., blaCTX-M-14, blaCTX-M-15, blaCTX-M-27, and blaCTX-M-55). Hierarchical clustering using selected phenotypic and genotypic characteristics suggested clonal dissemination of blaCTX-M genes. Out of 45 blaCTX-M gene-carrying strains, 7 harbored the gene in their chromosome. In one E. cloacae and three E. coli strains, chromosomal blaCTX-M-15 was located on insertions longer than 10 kb. These insertions were bounded by ISEcp1 at one end, exhibited a high degree of nucleotide sequence homology with previously reported plasmids, and carried multiple AMR genes that corresponded with phenotypic AMR profiles.
Conclusion: Our study revealed the co-occurrence of ISEcp1-blaCTX-M-15 and multiple AMR genes on chromosomal insertions in E. cloacae and E. coli, suggesting that ISEcp1 may be responsible for the transposition of diverse AMR genes from plasmids to chromosomes. Stable retention of such insertions in chromosomes may facilitate the successful propagation of MDR clones among these Enterobacteriaceae species.
Keywords: AMR; Chromosomal insertion; Enterobacter cloacae; Escherichia coli; Extended spectrum β-lactamase; ISEcp1; Zambia; bla CTX-M.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Hendriksen RS, Munk P, Njage P, van Bunnik B, McNally L, Lukjancenko O, Röder T, Nieuwenhuijse D, Pedersen SK, Kjeldgaard J, et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun. 2019;10:1124. doi: 10.1038/s41467-019-08853-3. - DOI - PMC - PubMed
-
- Zerr DM, Miles-Jay A, Kronman MP, Zhou C, Adler AL, Haaland W, Weissman SJ, Elward A, Newland JG, Zaoutis T, Qin X. Previous antibiotic exposure increases risk of infection with extended-spectrum-β-Lactamase- and AmpC-producing Escherichia coli and Klebsiella pneumoniae in Pediatric Patients. Antimicrob Agents Chemother. 2016;60:4237–4243. doi: 10.1128/AAC.00187-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

