Hydroxyethyl starch 130/0.4 for volume replacement therapy in surgical patients: a systematic review and meta-analysis of randomized controlled trials
- PMID: 33971968
- PMCID: PMC8111748
- DOI: 10.1186/s13741-021-00182-8
Hydroxyethyl starch 130/0.4 for volume replacement therapy in surgical patients: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: The safety of perioperative intravenous hydroxyethyl starch (HES) products, specifically HES 130/0.4, continues to be the source of much debate. The aim of this meta-analysis was to update the existing evidence and gain further insight into the clinical effects of HES 130/0.4 on postoperative outcomes for volume replacement therapy in surgical patients.
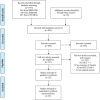
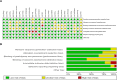
Methods: MEDLINE, EMBASE, and Cochrane Library databases were searched from inception to March 2020 for relevant randomized controlled trials (RCTs) on perioperative use of HES 130/0.4 in adult surgical patients. The primary outcome was postoperative mortality and secondary outcomes were the incidence of acute kidney injury (AKI) and requirement for renal replacement therapy (RRT). The analysis was performed using the random-effects method and the risk ratio (RR) with a 95% confidence interval (CI). We performed the risk-of-bias assessment of eligible studies and assessed the overall quality of evidence for each outcome.
Results: Twenty-five RCTs with 4111 participants were finally included. There were no statistical differences between HES 130/0.4 and other fluids in mortality at 30 days (RR 1.28, 95% CI 0.88 to 1.86, p = 0.20), the incidence of AKI (RR 1.23, 95% CI 0.99 to 1.53, p = 0.07), or requirement for RRT (RR 0.75, 95% CI 0.37 to 1.53, p = 0.43). Overall, there was a moderate certainty of evidence for all the outcomes. There was no subgroup difference related to the type of surgery (p = 0.17) in the incidence of AKI. As for the type of comparator fluids, however, there was a trend that was not statistically significant (p = 0.06) towards the increased incidence of AKI in the HES 130/0.4 group (RR 1.22, 95% CI 0.97 to 1.54) compared with the crystalloid group (RR 1.21, 95% CI 0.27 to 3.91). Subgroup analyses according to the type of surgery demonstrated consistent findings.
Conclusions: This systematic review and meta-analysis suggests that the use of HES 130/0.4 for volume replacement therapy compared with other fluids resulted in no significant difference in postoperative mortality or kidney dysfunction among surgical patients. Given the absent evidence of confirmed benefit and the potential trend of increased kidney injury, we cannot recommend the routine clinical use of HES 130/0.4 for volume replacement therapy in surgical patients from the perspective of benefit/risk profile. However, the results need to be interpreted with caution due to the limited sample size, and further well-powered RCTs are warranted.
Trial registration: PROSPERO registry reference: CRD42020173058.
Keywords: Hydroxyethyl starch 130/0.4; Surgery; Volume replacement therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. - DOI - PMC - PubMed
-
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39. 10.1056/NEJMoa070716. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

