Amikacin Combined with Fosfomycin for Treatment of Neonatal Sepsis in the Setting of Highly Prevalent Antimicrobial Resistance
- PMID: 33972238
- PMCID: PMC8373250
- DOI: 10.1128/AAC.00293-21
Amikacin Combined with Fosfomycin for Treatment of Neonatal Sepsis in the Setting of Highly Prevalent Antimicrobial Resistance
Abstract
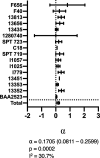
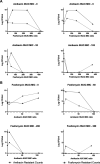
Antimicrobial resistance (particularly through extended-spectrum β-lactamase and aminoglycoside-modifying enzyme production) in neonatal sepsis is a global problem, particularly in low- and middle-income countries, with significant mortality rates. High rates of resistance are reported for the current WHO-recommended first-line antibiotic regimen for neonatal sepsis, i.e., ampicillin and gentamicin. We assessed the utility of fosfomycin and amikacin as a potential alternative regimen to be used in settings of increasingly prevalent antimicrobial resistance. The combination was studied in a 16-arm dose-ranged hollow-fiber infection model (HFIM) experiment. The combination of amikacin and fosfomycin enhanced bactericidal activity and prevented the emergence of resistance, compared to monotherapy with either antibiotic. Modeling of the experimental quantitative outputs and data from checkerboard assays indicated synergy. We further assessed the combination regimen at clinically relevant doses in the HFIM with nine Enterobacterales strains with high fosfomycin and amikacin MICs and demonstrated successful kill to sterilization for 6/9 strains. From these data, we propose a novel combination breakpoint threshold for microbiological success for this antimicrobial combination against Enterobacterales strains, i.e., MICF × MICA < 256 (where MICF and MICA are the fosfomycin and amikacin MICs, respectively). Monte Carlo simulations predict that a standard fosfomycin-amikacin neonatal regimen would achieve >99% probability of pharmacodynamic success for strains with MICs below this threshold. We conclude that the combination of fosfomycin with amikacin is a viable regimen for the empirical treatment of neonatal sepsis and is suitable for further clinical assessment in a randomized controlled trial.
Keywords: amikacin; aminoglycosides; antimicrobial resistance; combination antibiotics; fosfomycin; hollow fiber; mathematical modelling; neonatal sepsis; pharmacodynamics; synergy.
Figures


Similar articles
-
Flomoxef and fosfomycin in combination for the treatment of neonatal sepsis in the setting of highly prevalent antimicrobial resistance.J Antimicrob Chemother. 2022 Apr 27;77(5):1334-1343. doi: 10.1093/jac/dkac038. J Antimicrob Chemother. 2022. PMID: 35170719 Free PMC article.
-
Assessment of flomoxef combined with amikacin in a hollow-fibre infection model for the treatment of neonatal sepsis in low- and middle-income healthcare settings.J Antimicrob Chemother. 2022 Nov 28;77(12):3349-3357. doi: 10.1093/jac/dkac323. J Antimicrob Chemother. 2022. PMID: 36177766 Free PMC article.
-
Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS).Lancet Infect Dis. 2021 Dec;21(12):1677-1688. doi: 10.1016/S1473-3099(21)00050-5. Epub 2021 Aug 9. Lancet Infect Dis. 2021. PMID: 34384533 Free PMC article.
-
Potential Antibiotics for the Treatment of Neonatal Sepsis Caused by Multidrug-Resistant Bacteria.Paediatr Drugs. 2021 Sep;23(5):465-484. doi: 10.1007/s40272-021-00465-z. Epub 2021 Aug 26. Paediatr Drugs. 2021. PMID: 34435316 Free PMC article. Review.
-
Literature review on the distribution characteristics and antimicrobial resistance of bacterial pathogens in neonatal sepsis.J Matern Fetal Neonatal Med. 2022 Mar;35(5):861-870. doi: 10.1080/14767058.2020.1732342. Epub 2020 Feb 26. J Matern Fetal Neonatal Med. 2022. PMID: 32102584 Review.
Cited by
-
Early-Onset Neonatal Sepsis in Low- and Middle-Income Countries: Current Challenges and Future Opportunities.Infect Drug Resist. 2022 Mar 9;15:933-946. doi: 10.2147/IDR.S294156. eCollection 2022. Infect Drug Resist. 2022. PMID: 35299860 Free PMC article. Review.
-
Reintroduction of Legacy Antibiotics in Neonatal Sepsis: The Special Role of Fosfomycin and Colistin.Antibiotics (Basel). 2024 Apr 5;13(4):333. doi: 10.3390/antibiotics13040333. Antibiotics (Basel). 2024. PMID: 38667009 Free PMC article. Review.
-
Emerging Concepts for the Treatment of Biofilm-Associated Bone and Joint Infections with IV Fosfomycin: A Literature Review.Microorganisms. 2025 Apr 23;13(5):963. doi: 10.3390/microorganisms13050963. Microorganisms. 2025. PMID: 40431135 Free PMC article. Review.
-
Bibliometric analysis of global research on the clinical applications of aminoglycoside antibiotics: improving efficacy and decreasing risk.Front Microbiol. 2025 Feb 19;16:1532231. doi: 10.3389/fmicb.2025.1532231. eCollection 2025. Front Microbiol. 2025. PMID: 40046300 Free PMC article.
-
Flomoxef and fosfomycin in combination for the treatment of neonatal sepsis in the setting of highly prevalent antimicrobial resistance.J Antimicrob Chemother. 2022 Apr 27;77(5):1334-1343. doi: 10.1093/jac/dkac038. J Antimicrob Chemother. 2022. PMID: 35170719 Free PMC article.
References
-
- Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, Zaidi AK, Berkley JA, Cousens SN, Lawn JE. 2014. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis 14:731–741. doi:10.1016/S1473-3099(14)70804-7. - DOI - PMC - PubMed
-
- Fuchs A, Bielicki J, Mathur S, Sharland M, Van Den Anker JN. 2016. Antibiotic use for sepsis in neonates and children: 2016 evidence update. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2013. Pocket book of hospital care for children, 2nd ed. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

