Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (HADS-D) to screen for major depression: systematic review and individual participant data meta-analysis
- PMID: 33972268
- PMCID: PMC8107836
- DOI: 10.1136/bmj.n972
Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (HADS-D) to screen for major depression: systematic review and individual participant data meta-analysis
Erratum in
-
Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (HADS-D) to screen for major depression: systematic review and individual participant data meta-analysis.BMJ. 2021 May 19;373:n1231. doi: 10.1136/bmj.n1231. BMJ. 2021. PMID: 34011511 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the accuracy of the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D) to screen for major depression among people with physical health problems.
Design: Systematic review and individual participant data meta-analysis.
Data sources: Medline, Medline In-Process and Other Non-Indexed Citations, PsycInfo, and Web of Science (from inception to 25 October 2018).
Review methods: Eligible datasets included HADS-D scores and major depression status based on a validated diagnostic interview. Primary study data and study level data extracted from primary reports were combined. For HADS-D cut-off thresholds of 5-15, a bivariate random effects meta-analysis was used to estimate pooled sensitivity and specificity, separately, in studies that used semi-structured diagnostic interviews (eg, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders), fully structured interviews (eg, Composite International Diagnostic Interview), and the Mini International Neuropsychiatric Interview. One stage meta-regression was used to examine whether accuracy was associated with reference standard categories and the characteristics of participants. Sensitivity analyses were done to assess whether including published results from studies that did not provide raw data influenced the results.
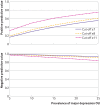
Results: Individual participant data were obtained from 101 of 168 eligible studies (60%; 25 574 participants (72% of eligible participants), 2549 with major depression). Combined sensitivity and specificity was maximised at a cut-off value of seven or higher for semi-structured interviews, fully structured interviews, and the Mini International Neuropsychiatric Interview. Among studies with a semi-structured interview (57 studies, 10 664 participants, 1048 with major depression), sensitivity and specificity were 0.82 (95% confidence interval 0.76 to 0.87) and 0.78 (0.74 to 0.81) for a cut-off value of seven or higher, 0.74 (0.68 to 0.79) and 0.84 (0.81 to 0.87) for a cut-off value of eight or higher, and 0.44 (0.38 to 0.51) and 0.95 (0.93 to 0.96) for a cut-off value of 11 or higher. Accuracy was similar across reference standards and subgroups and when published results from studies that did not contribute data were included.
Conclusions: When screening for major depression, a HADS-D cut-off value of seven or higher maximised combined sensitivity and specificity. A cut-off value of eight or higher generated similar combined sensitivity and specificity but was less sensitive and more specific. To identify medically ill patients with depression with the HADS-D, lower cut-off values could be used to avoid false negatives and higher cut-off values to reduce false positives and identify people with higher symptom levels.
Trial registration: PROSPERO CRD42015016761.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICJME uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years with the following exceptions: ZI declares that he has received personal fees from Avanir, Janssen, Lundbeck, Otsuka, and Sunovion, outside the submitted work. MTonelli declares that he has received a grant from Merck Canada, outside the submitted work. CNB declares that he has consulted for Abbvie Canada, Amgen Canada, Bristol Myers Squibb Canada, Roche Canada, Janssen Canada, Pfizer Canada, Sandoz Canada, Takeda Canada, and Mylan Pharmaceuticals. He has also received unrestricted educational grants from Abbvie Canada, Janssen Canada, Pfizer Canada, and Takeda Canada; as well as been on speaker's bureau of Abbvie Canada, Janssen Canada, Takeda Canada, and Medtronic Canada, all outside the submitted work. AF reports that he received speaker's honorariums from Biogen, Sanofi-Genzyme, Merck-Serono, Novartis, and Roche, and is on the advisory board for Akili Interactive, outside the submitted work; he has also received royalties from the Cambridge University Press for the Clinical Neuropsychiatry of Multiple Sclerosis, 2nd edition. BLöwe declares that the primary study by Löwe et al was supported by unrestricted educational grants from Pfizer, Germany. RAM declares that she has conducted clinical trials for Sanofi Aventis, outside the submitted work. YM declares that he has received personal fees from Mochida, Pfizer, Eli Lilly, Morinaga Milk, and NTT Data, outside the submitted work. SSinger declares that she has received personal fees from Lilly, BMS, and Pfizer, outside the submitted work. JStone declares that he has received personal fees from UptoDate, outside the submitted work. SSultan declares funding from Sanofi-Aventis Corporation, during conduct of the primary study. All authors declare no other relationships or activities that could appear to have influenced the submitted work. No funder had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures


Comment in
-
Accuracy of depression score: a positive HADS score may only be the tip of the iceberg.BMJ. 2021 Jun 17;373:n1541. doi: 10.1136/bmj.n1541. BMJ. 2021. PMID: 34140273 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
