uPAR+ extracellular vesicles: a robust biomarker of resistance to checkpoint inhibitor immunotherapy in metastatic melanoma patients
- PMID: 33972390
- PMCID: PMC8112420
- DOI: 10.1136/jitc-2021-002372
uPAR+ extracellular vesicles: a robust biomarker of resistance to checkpoint inhibitor immunotherapy in metastatic melanoma patients
Abstract
Background: Emerging evidence has highlighted the importance of extracellular vesicle (EV)-based biomarkers of resistance to immunotherapy with checkpoint inhibitors in metastatic melanoma. Considering the tumor-promoting implications of urokinase-type plasminogen activator receptor (uPAR) signaling, this study aimed to assess uPAR expression in the plasma-derived EVs of patients with metastatic melanoma to determine its potential correlation with clinical outcomes.
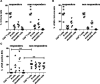
Methods: Blood samples from 71 patients with metastatic melanoma were collected before initiating immunotherapy. Tumor-derived and immune cell-derived EVs were isolated and analyzed to assess the relative percentage of uPAR+ EVs. The associations between uPAR and clinical outcomes, sex, BRAF status, baseline lactate dehydrogenase levels and number of metastatic sites were assessed.
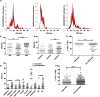
Results: Responders had a significantly lower percentage of tumor-derived, dendritic cell (DC)-derived and CD8+ T cell-derived uPAR +EVs at baseline than non-responders. The Kaplan-Meier survival curves for the uPAR+EV quartiles indicated that higher levels of melanoma-derived uPAR+ EVs were strongly correlated with poorer progression-free survival (p<0.0001) and overall survival (p<0.0001). We also found a statistically significant correlation between lower levels of uPAR+ EVs from both CD8+ T cells and DCs and better survival.
Conclusions: Our results indicate that higher levels of tumor-derived, DC-derived and CD8+ T cell-derived uPAR+ EVs in non-responders may represent a new biomarker of innate resistance to immunotherapy with checkpoint inhibitors. Moreover, uPAR+ EVs represent a new potential target for future therapeutic approaches.
Keywords: Biomarkers; CD8-Positive T-Lymphocytes; Dendritic Cells; Immunotherapy; Melanoma; Tumor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Anon . Abstracts. Pigment Cell Melanoma Res 2020;33:148–255. 10.1111/pcmr.12834 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
