Erf Affects Commitment and Differentiation of Osteoprogenitor Cells in Cranial Sutures via the Retinoic Acid Pathway
- PMID: 33972395
- PMCID: PMC8300784
- DOI: 10.1128/MCB.00149-21
Erf Affects Commitment and Differentiation of Osteoprogenitor Cells in Cranial Sutures via the Retinoic Acid Pathway
Abstract
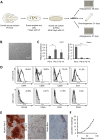
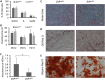
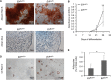
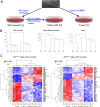
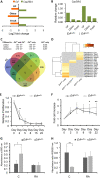
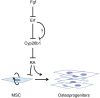
ETS2 repressor factor (ERF) haploinsufficiency causes late-onset craniosynostosis (CRS) (OMIM entry 600775; CRS4) in humans, while in mice Erf insufficiency also leads to a similar multisuture synostosis phenotype preceded by mildly reduced calvarium ossification. However, neither the cell types affected nor the effects per se have been identified so far. Here, we establish an ex vivo system for the expansion of suture-derived mesenchymal stem and progenitor cells (sdMSCs) and analyze the role of Erf levels in their differentiation. Cellular data suggest that Erf insufficiency specifically decreases osteogenic differentiation of sdMSCs, resulting in the initially delayed mineralization of the calvarium. Transcriptome analysis indicates that Erf is required for efficient osteogenic lineage commitment of sdMSCs. Elevated retinoic acid catabolism due to increased levels of the cytochrome P450 superfamily member Cyp26b1 as a result of decreased Erf levels appears to be the underlying mechanism leading to defective differentiation. Exogenous addition of retinoic acid can rescue the osteogenic differentiation defect, suggesting that Erf affects cranial bone mineralization during skull development through retinoic acid gradient regulation.
Keywords: Ets transcription factors; craniosynostosis; mesenchymal stem cells; retinoic acid.
Figures


References
-
- Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, Li N, Liu Y, Yang YS, Eiseman M, Shim JH, Hameed M, Healey JH, Bostrom MP, Landau DA, Greenblatt MB. 2018. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562:133–139. 10.1038/s41586-018-0554-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
