Structural basis of the protochromic green/red photocycle of the chromatic acclimation sensor RcaE
- PMID: 33972439
- PMCID: PMC8157938
- DOI: 10.1073/pnas.2024583118
Structural basis of the protochromic green/red photocycle of the chromatic acclimation sensor RcaE
Abstract
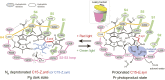
Cyanobacteriochromes (CBCRs) are bilin-binding photosensors of the phytochrome superfamily that show remarkable spectral diversity. The green/red CBCR subfamily is important for regulating chromatic acclimation of photosynthetic antenna in cyanobacteria and is applied for optogenetic control of gene expression in synthetic biology. It is suggested that the absorption change of this subfamily is caused by the bilin C15-Z/C15-E photoisomerization and a subsequent change in the bilin protonation state. However, structural information and direct evidence of the bilin protonation state are lacking. Here, we report a high-resolution (1.63Å) crystal structure of the bilin-binding domain of the chromatic acclimation sensor RcaE in the red-absorbing photoproduct state. The bilin is buried within a "bucket" consisting of hydrophobic residues, in which the bilin configuration/conformation is C5-Z,syn/C10-Z,syn/C15-E,syn with the A- through C-rings coplanar and the D-ring tilted. Three pyrrole nitrogens of the A- through C-rings are covered in the α-face with a hydrophobic lid of Leu249 influencing the bilin pKa, whereas they are directly hydrogen bonded in the β-face with the carboxyl group of Glu217. Glu217 is further connected to a cluster of waters forming a hole in the bucket, which are in exchange with solvent waters in molecular dynamics simulation. We propose that the "leaky bucket" structure functions as a proton exit/influx pathway upon photoconversion. NMR analysis demonstrated that the four pyrrole nitrogen atoms are indeed fully protonated in the red-absorbing state, but one of them, most likely the B-ring nitrogen, is deprotonated in the green-absorbing state. These findings deepen our understanding of the diverse spectral tuning mechanisms present in CBCRs.
Keywords: NMR; crystallography; cyanobacteriochrome; phytochrome.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Probing Bilin-Protein Interaction in the Protochromic Photocycle of Cyanobacteriochrome RcaE by Site-Directed Mutagenesis.Plant Cell Physiol. 2025 Feb 28;66(2):181-192. doi: 10.1093/pcp/pcae085. Plant Cell Physiol. 2025. PMID: 39092561
-
Green/red cyanobacteriochromes regulate complementary chromatic acclimation via a protochromic photocycle.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):4974-9. doi: 10.1073/pnas.1302909110. Epub 2013 Mar 11. Proc Natl Acad Sci U S A. 2013. PMID: 23479641 Free PMC article.
-
Deprotonation at Ring B Is an Intrinsic Property of the Bilin Chromophore in Cyanobacteriochrome RcaE.J Phys Chem B. 2025 Mar 20;129(11):2986-2991. doi: 10.1021/acs.jpcb.5c00744. Epub 2025 Mar 11. J Phys Chem B. 2025. PMID: 40070126
-
Cyanobacteriochromes: A Rainbow of Photoreceptors.Annu Rev Microbiol. 2024 Nov;78(1):61-81. doi: 10.1146/annurev-micro-041522-094613. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 38848579 Free PMC article. Review.
-
A brief history of phytochromes.Chemphyschem. 2010 Apr 26;11(6):1172-80. doi: 10.1002/cphc.200900894. Chemphyschem. 2010. PMID: 20155775 Free PMC article. Review.
Cited by
-
Enhancing the Inhomogeneous Photodynamics of Canonical Bacteriophytochrome.J Phys Chem B. 2022 Apr 14;126(14):2647-2657. doi: 10.1021/acs.jpcb.2c00131. Epub 2022 Mar 31. J Phys Chem B. 2022. PMID: 35357137 Free PMC article.
-
Crystal structure of a far-red-sensing cyanobacteriochrome reveals an atypical bilin conformation and spectral tuning mechanism.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2025094118. doi: 10.1073/pnas.2025094118. Proc Natl Acad Sci U S A. 2021. PMID: 33727422 Free PMC article.
-
Light- and pH-dependent structural changes in cyanobacteriochrome AnPixJg2.Photochem Photobiol Sci. 2022 Apr;21(4):447-469. doi: 10.1007/s43630-022-00204-4. Epub 2022 Apr 8. Photochem Photobiol Sci. 2022. PMID: 35394641
-
Distinct Protochromic Mechanisms Driving Green/Red Absorption in Phycocyanobilin-Binding Proteins.Biochemistry. 2025 Jul 1;64(13):2823-2833. doi: 10.1021/acs.biochem.4c00870. Epub 2025 Jun 18. Biochemistry. 2025. PMID: 40532088
-
Direct evidence for a deprotonated lysine serving as a H-bond "acceptor" in a photoreceptor protein.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2404472121. doi: 10.1073/pnas.2404472121. Epub 2024 Aug 27. Proc Natl Acad Sci U S A. 2024. PMID: 39190358 Free PMC article.
References
-
- Ikeuchi M., Ishizuka T., Cyanobacteriochromes: A new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 7, 1159–1167 (2008). - PubMed
-
- Auldridge M. E., Forest K. T., Bacterial phytochromes: More than meets the light. Crit. Rev. Biochem. Mol. Biol. 46, 67–88 (2011). - PubMed
-
- Wiltbank L. B., Kehoe D. M., Diverse light responses of cyanobacteria mediated by phytochrome superfamily photoreceptors. Nat. Rev. Microbiol. 17, 37–50 (2019). - PubMed
-
- Fushimi K., Narikawa R., Cyanobacteriochromes: Photoreceptors covering the entire UV-to-visible spectrum. Curr. Opin. Struct. Biol. 57, 39–46 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

