Hydroxyurea-induced membrane fluidity decreasing as a characterization of neuronal membrane aging in Alzheimer's disease
- PMID: 33972461
- PMCID: PMC8148445
- DOI: 10.18632/aging.202949
Hydroxyurea-induced membrane fluidity decreasing as a characterization of neuronal membrane aging in Alzheimer's disease
Abstract
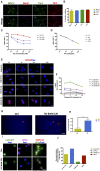
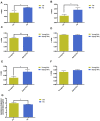
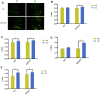
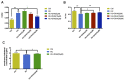
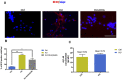
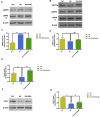
Aging is one of the significant risk factors for Alzheimer's disease (AD). Therefore, this study aimed to propose a new hypothesis "membrane aging" as a critical pathogenesis of AD. The concept of "membrane aging" was reviewed, and the possible mechanisms of membrane aging as the primary culprit of AD were clarified. To further prove this hypothesis, a hydroxyurea-induced "membrane aging" model was established in vitro and in vivo. First, neuronal aging was validated by immunocytochemistry with age-related markers, and membrane aging phenotypes were confirmed. The alterations of membrane fluidity within APP/PS1 mice were re-proved by intracerebroventricular injection of hydroxyurea. Decreased membrane fluidity was found in vitro and in vivo, accompanied by increased total cholesterol concentration in neurons but decreased cholesterol levels within membrane fractions. The Aβ level increased considerably after hydroxyurea treatment both in vitro and in vivo. DHA co-treatment ameliorated membrane aging phenotypes and Aβ aggregation. The study revealed the AMP-activated protein kinase/acetyl CoA carboxylase/carnitine palmitoyl transferase 1 pathway involved in membrane aging processes. These results strongly supported the idea that membrane aging was a pathogenesis of AD and might serve as a new therapeutic target for AD.
Keywords: Alzheimer's disease; DHA; hydroxyurea; membrane aging; β-amyloid.
Conflict of interest statement
Figures
Similar articles
-
The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer's disease.Brain Res. 2018 Jul 15;1691:87-93. doi: 10.1016/j.brainres.2018.03.034. Epub 2018 Apr 3. Brain Res. 2018. PMID: 29625119
-
Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease.Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):2070-5. doi: 10.1073/pnas.0305799101. Epub 2004 Feb 15. Proc Natl Acad Sci U S A. 2004. PMID: 14970312 Free PMC article.
-
Amyloid β Protein Aggravates Neuronal Senescence and Cognitive Deficits in 5XFAD Mouse Model of Alzheimer's Disease.Chin Med J (Engl). 2016 Aug 5;129(15):1835-44. doi: 10.4103/0366-6999.186646. Chin Med J (Engl). 2016. PMID: 27453234 Free PMC article.
-
Cholesterol, lipids, amyloid Beta, and Alzheimer's.Curr Alzheimer Res. 2010 May;7(3):262-70. doi: 10.2174/156720510791050821. Curr Alzheimer Res. 2010. PMID: 19715550 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
P2X7-mediated alteration of membrane fluidity is associated with the late stages of age-related macular degeneration.Purinergic Signal. 2022 Dec;18(4):469-479. doi: 10.1007/s11302-022-09894-y. Epub 2022 Aug 24. Purinergic Signal. 2022. PMID: 36001279 Free PMC article.
-
Dynamic Progress in Technological Advances to Study Lipids in Aging: Challenges and Future Directions.Front Aging. 2022 Mar 10;3:851073. doi: 10.3389/fragi.2022.851073. eCollection 2022. Front Aging. 2022. PMID: 35821837 Free PMC article. Review.
-
mARC1 Is the Main Contributor to Metabolic Reduction of N-Hydroxyurea.J Med Chem. 2024 Oct 24;67(20):18090-18097. doi: 10.1021/acs.jmedchem.4c01148. Epub 2024 Oct 13. J Med Chem. 2024. PMID: 39397364 Free PMC article.
-
Cannabinoid receptor type 1 in the aging gut regulates the mucosal permeability via miR-191-5p.Front Endocrinol (Lausanne). 2023 Aug 25;14:1241097. doi: 10.3389/fendo.2023.1241097. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37693348 Free PMC article.
-
Informing the Cannabis Conjecture: From Life's Beginnings to Mitochondria, Membranes and the Electrome-A Review.Int J Mol Sci. 2023 Aug 22;24(17):13070. doi: 10.3390/ijms241713070. Int J Mol Sci. 2023. PMID: 37685877 Free PMC article. Review.
References
-
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I, and Global Health Epidemiology Reference Group (GHERG). Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet. 2013; 381:2016–23. 10.1016/S0140-6736(13)60221-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

