Breathing-driven prefrontal oscillations regulate maintenance of conditioned-fear evoked freezing independently of initiation
- PMID: 33972521
- PMCID: PMC8110519
- DOI: 10.1038/s41467-021-22798-6
Breathing-driven prefrontal oscillations regulate maintenance of conditioned-fear evoked freezing independently of initiation
Abstract
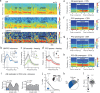
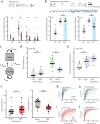
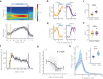
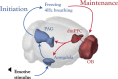
Brain-body interactions are thought to be essential in emotions but their physiological basis remains poorly understood. In mice, regular 4 Hz breathing appears during freezing after cue-fear conditioning. Here we show that the olfactory bulb (OB) transmits this rhythm to the dorsomedial prefrontal cortex (dmPFC) where it organizes neural activity. Reduction of the respiratory-related 4 Hz oscillation, via bulbectomy or optogenetic perturbation of the OB, reduces freezing. Behavioural modelling shows that this is due to a specific reduction in freezing maintenance without impacting its initiation, thus dissociating these two phenomena. dmPFC LFP and firing patterns support the region's specific function in freezing maintenance. In particular, population analysis reveals that network activity tracks 4 Hz power dynamics during freezing and reaches a stable state at 4 Hz peak that lasts until freezing termination. These results provide a potential mechanism and a functional role for bodily feedback in emotions and therefore shed light on the historical James-Cannon debate.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Subramanian, H. H. & Holstege, G. in Progress in Brain Research, Vol. 212, 351–384 (Elsevier B.V., 2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

