RHOA signaling defects result in impaired axon guidance in iPSC-derived neurons from patients with tuberous sclerosis complex
- PMID: 33972524
- PMCID: PMC8110792
- DOI: 10.1038/s41467-021-22770-4
RHOA signaling defects result in impaired axon guidance in iPSC-derived neurons from patients with tuberous sclerosis complex
Abstract
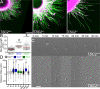
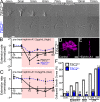
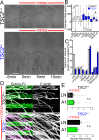
Patients with Tuberous Sclerosis Complex (TSC) show aberrant wiring of neuronal connections formed during development which may contribute to symptoms of TSC, such as intellectual disabilities, autism, and epilepsy. Yet models examining the molecular basis for axonal guidance defects in developing human neurons have not been developed. Here, we generate human induced pluripotent stem cell (hiPSC) lines from a patient with TSC and genetically engineer counterparts and isogenic controls. By differentiating hiPSCs, we show that control neurons respond to canonical guidance cues as predicted. Conversely, neurons with heterozygous loss of TSC2 exhibit reduced responses to several repulsive cues and defective axon guidance. While TSC2 is a known key negative regulator of MTOR-dependent protein synthesis, we find that TSC2 signaled through MTOR-independent RHOA in growth cones. Our results suggest that neural network connectivity defects in patients with TSC may result from defects in RHOA-mediated regulation of cytoskeletal dynamics during neuronal development.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Tuberous Sclerosis Complex (TSC) Inactivation Increases Neuronal Network Activity by Enhancing Ca2+ Influx via L-Type Ca2+ Channels.J Neurosci. 2021 Sep 29;41(39):8134-8149. doi: 10.1523/JNEUROSCI.1930-20.2021. Epub 2021 Aug 20. J Neurosci. 2021. PMID: 34417327 Free PMC article.
-
Pharmacological intervention to restore connectivity deficits of neuronal networks derived from ASD patient iPSC with a TSC2 mutation.Mol Autism. 2020 Oct 19;11(1):80. doi: 10.1186/s13229-020-00391-w. Mol Autism. 2020. PMID: 33076974 Free PMC article.
-
Genetic Etiologies, Diagnosis, and Treatment of Tuberous Sclerosis Complex.Annu Rev Genomics Hum Genet. 2019 Aug 31;20:217-240. doi: 10.1146/annurev-genom-083118-015354. Epub 2019 Apr 24. Annu Rev Genomics Hum Genet. 2019. PMID: 31018109 Review.
-
Biallelic Mutations in TSC2 Lead to Abnormalities Associated with Cortical Tubers in Human iPSC-Derived Neurons.J Neurosci. 2019 Nov 20;39(47):9294-9305. doi: 10.1523/JNEUROSCI.0642-19.2019. Epub 2019 Oct 7. J Neurosci. 2019. PMID: 31591157 Free PMC article.
-
The role of mTOR signalling in neurogenesis, insights from tuberous sclerosis complex.Semin Cell Dev Biol. 2016 Apr;52:12-20. doi: 10.1016/j.semcdb.2016.01.040. Epub 2016 Feb 2. Semin Cell Dev Biol. 2016. PMID: 26849906 Free PMC article. Review.
Cited by
-
Epileptogenesis in tuberous sclerosis complex-related developmental and epileptic encephalopathy.Brain. 2023 Jul 3;146(7):2694-2710. doi: 10.1093/brain/awad048. Brain. 2023. PMID: 36806388 Free PMC article. Review.
-
A whole transcriptome profiling analysis for antidepressant mechanism of Xiaoyaosan mediated synapse loss via BDNF/trkB/PI3K signal axis in CUMS rats.BMC Complement Med Ther. 2023 Jun 15;23(1):198. doi: 10.1186/s12906-023-04000-0. BMC Complement Med Ther. 2023. PMID: 37322430 Free PMC article.
-
Mechanistic target of rapamycin signaling in human nervous system development and disease.Front Mol Neurosci. 2022 Sep 26;15:1005631. doi: 10.3389/fnmol.2022.1005631. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36226315 Free PMC article. Review.
-
Delayed forebrain excitatory and inhibitory neurogenesis in STRADA-related megalencephaly via mTOR hyperactivity.bioRxiv [Preprint]. 2025 May 14:2025.05.13.653911. doi: 10.1101/2025.05.13.653911. bioRxiv. 2025. PMID: 40462897 Free PMC article. Preprint.
-
Molecular Mechanisms of Alzheimer's Disease Induced by Amyloid-β and Tau Phosphorylation Along with RhoA Activity: Perspective of RhoA/Rho-Associated Protein Kinase Inhibitors for Neuronal Therapy.Cells. 2025 Jan 10;14(2):89. doi: 10.3390/cells14020089. Cells. 2025. PMID: 39851517 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

