Bortezomib induces methylation changes in neuroblastoma cells that appear to play a significant role in resistance development to this compound
- PMID: 33972578
- PMCID: PMC8110815
- DOI: 10.1038/s41598-021-89128-0
Bortezomib induces methylation changes in neuroblastoma cells that appear to play a significant role in resistance development to this compound
Abstract
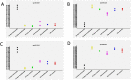
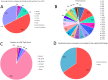
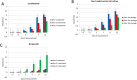
The anticancer activity of bortezomib (BTZ) has been increasingly studied in a number of indications and promising results for the use of this treatment have been shown in neuroblastoma. As BTZ treatment is usually administered in cycles, the development of resistance and side effects in patients undergoing therapy with BTZ remains a major challenge for the clinical usage of this compound. Common resistance development also means that certain cells are able to survive BTZ treatment and bypass molecular mechanisms that render BTZ anticancer activity. We studied the methylome of neuroblastoma cells that survived BTZ treatment. Our results indicate that BTZ induces pronounced genome wide methylation changes in cells which recovered from the treatment. Functional analyses of identified methylation changes demonstrated they were involved in key cancer pathology pathways. These changes may allow the cells to bypass the primary anticancer activity of BTZ and develop a treatment resistant and proliferative phenotype. To study whether cells surviving BTZ treatment acquire a proliferative phenotype, we repeatedly treated cells which recovered from the first round of BTZ treatment. The repetitive treatment led to induction of the extraordinary proliferative potential of the cells, that increased with subsequent treatments. As we did not observe similar effects in cells that survived treatment with lenalidomide, and non-treated cells cultured under the same experimental conditions, this phenomenon seems to be BTZ specific. Overall, our results indicate that methylation changes may play major role in the development of BTZ resistance.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

